
3D printing technology is a globally emerging technology in the field of medicine which has a great potential of improving the quality of life and changing the way in which pharmaceuticals are manufactured. By layering material into a product, 3D printing technology generates complex geometric structures through a digitally controlled process.
3D printing technology, also known as additive manufacturing, is a digitally-controlled technique of fabricating a product by layer-wise addition of the feed material to generate complex geometric structures. This technique has wide applications in the field of mechanics, consumer goods, electronics, aeronautics, medicine, the food industry, and various other fronts.
The manufacturing process of pharmaceuticals has progressed from batch process to continuous process and now to printing.3D printing technology started gaining increased attention in the pharmaceutical field after the USFDA approval of the first 3D printed pill Spritam® by Aprecia Pharmaceuticals in 2015. This technology has been utilized for the printing of medical devices, dental implants, artificial organs, research prototypes, tailored dosage forms, drug fabrication, and specialty surgical instruments. It offers great flexibility which justifies its use in a wide range of settings including educational institutions, hospitals, and even households. Globally, numerous industry experts have predicted the use of 3D printing technology for centralized manufacturing of pharmaceuticals, veterinary medicine, and in the early phases of clinical trials over the span of the next 5 years.
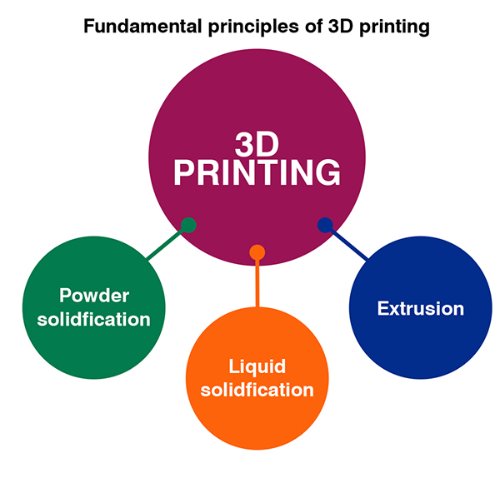
3D printing of pharmaceuticals can be carried out using feed materials like particles, particle colloids, plastic powders, polymers, polymer solutions, gels, polymer-particle composites, or continuous thin sheets.
1. Powder solidification is carried out by the techniques like selective laser sintering and binder jetting.
2. Liquid solidification is carried out by the techniques of stereolithography and inkjet printing.
3. Extrusion of the feed material is carried out by techniques of fused deposition modeling and semisolid extrusion.
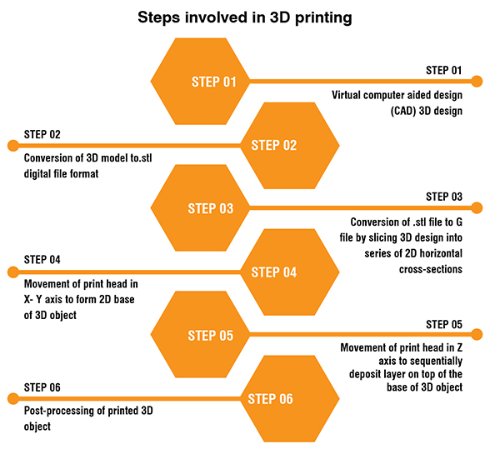
3D printing starts with the creation of a virtual 3D design of the object using digital design software like AutoCAD, SolidWorks, Autodesk, etc. The steps involved in the 3D printing process are mentioned in the following figure.
3D printing is a promising technology to help achieve the goal of precision medicine and personalized therapy. Immediate-release tablets, chewable tablets, orodispersible films, solid self-emulsifying formulations, microneedles, and hydrogel patches are some of the dosage forms which can be fabricated using 3D printing. A polypill containing multiple drugs in the same dosage form can be printed which will avoid polypharmacy and improve patient compliance. Incompatible drugs can also be fabricated in the same dosage form. The release profiles of the multiple drugs can be modified by using the appropriate release-modifying polymers for the individual drugs. Customized implants and prosthetics can also be printed as per the individual patient’s needs.
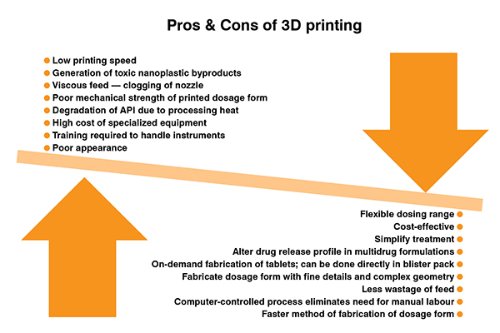
3D printing technology is a computer-controlled process that eliminates the need for manual labor thereby decreasing the incidences of human error in the process. This method minimizes the wastage of the feed material. It is capable of fabricating complex dosage forms and dosage forms containing multiple drugs with much ease. This simplifies the treatment therapy and increases patient compliance. The dosing range can be customized as per the needs of the individual patient. It is a faster and more convenient method of fabricating a dosage form. The dosage form can be printed and handed over to the patient at the point of care. On-demand fabrication of the dosage form can be done directly in the packing material itself which decreases the unit operations involved in the packaging of the dosage form. However, this technology can potentially generate toxic nanoplastic byproducts. The mechanical strength of the printed dosage form can be weak and has a poor appearance at times, which can affect patient compliance. The viscous feed material can sometimes lead to clogging of the printer nozzle in case of extrusion processes. The 3D printer is high-cost specialized equipment and requires trained personnel to handle the instrument.
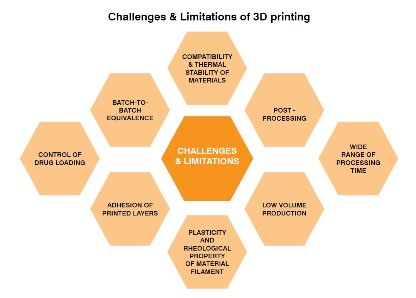
3D printing faces certain limitations and challenges to be used for manufacturing pharmaceuticals which have hindered the full-fledged large-scale usage of this technology for producing medicines. In the case of pharmaceuticals, there is a very limited number of materials that are compatible and suitable for the 3D printing process. The polymers used in formulating pharmaceutical dosage forms do not possess the desired mechanical strength, thermal stability, and rheological behavior to be suitable for 3D printing. The process of 3D printing is a low-volume production process and the printed dosage form or medical device requires postprocessing at times. In addition to this, there is a lack of batch-to-batch equivalence in the printed dosage form which poses a challenge to compliance with the regulatory guidelines.
Moving a step forward, 3D printing technology is further advancing to develop biopharmaceuticals by utilizing 4D printing technology. 4D printing technology is a modified form of 3D printing technology which uses stimuli-responsive materials, low-strength smart polymers, shapememory materials, self-healing materials, metals, and nanocomposites. Utilizing 4D printing technology, a real-time material response can be obtained. Such material is capable of responding to multiple environmental circumstances and stimuli which may be internal (pH, temperature, biomolecules) or external (magnetic field, ultrasound). The material can also produce a predictable response to an event or stimuli locally. By printing biocompatible materials or living cells (organ-on-chip), 4D bioprinting can serve as a means of fabricating biological structures that can respond to external stimulation by changing shape or functionality.A wide variety of applications for this technology exist, including drug delivery systems, wound therapies, tissue engineering, and organ regeneration.
The difference between 3D and 4D printing technology lies in the feed material utilized. 3D printing utilizes thermoplastics, powders, gels, nanomaterials, etc whereas 4D printing uses stimuli-responsive, shape-memory, and self-healing materials to fabricate dosage forms and medical devices. 3D printing utilizes digital information for the fabrication of a structure. On the other hand, 4D printing utilizes digital information for the deformation of a structure. The 4D printed product undergoes a change in its dimensions, structure, or appearance in response to a stimulus which does not occur in the case of most 3D printed products.
These advanced technologies need further development and optimization to overcome limitations concerning compliance with regulatory guidelines and large-scale manufacturing to increase their commercial applicability.
References:
1. Bandari S, Nyavanandi D, Dumpa N, Repka MA. Coupling hot melt extrusion and fused deposition modeling: Critical properties for successful performance. Advanced Drug Delivery Reviews. 2021, 52-63.
2. Mallakpour S, Tabesh F, Hussain CM. 3D and 4D printing: From innovation to evolution. Advances in Colloid and Interface Science. 2021, 102482.
3. Seoane-Viaño I, Trenfield SJ, Basit AW, Goyanes A. Translating 3D printed pharmaceuticals: From hype to real-world clinical applications. Advanced Drug Delivery Reviews. 2021, 553-575.
4. Okafor-Muo OL, Hassanin H, Kayyali R, ElShaer A. 3D Printing of Solid Oral Dosage Forms: Numerous Challenges With Unique Opportunities. Journal of Pharmaceutical Sciences. 2020.
5. Jamróz W, Szafraniec J, Kurek M, Jachowicz R. 3D Printing in Pharmaceutical and Medical Applications – Recent Achievements and Challenges. Pharmaceutical Research. 2018, 35:176.