Role of Artificial Intelligence and Machine Learning in Nanosafety
David Winkler, Professor of Biochemistry & Chemistry at La Trobe University, Professor of Pharmacy at the University of Nottingham, and Professor of Medicinal Chemistry at Monash University.
Medical applications of nanomaterials span drug, protein and vaccine delivery, diagnostics, theranostics. There is a need for a ‘safe-by-design’ paradigm for nanomaterials, and machine learning is increasingly used to predict their properties. I briefly review nanomaterials machine learning modelling and provide examples where model predictions have been validated by experiments.
Nanomaterials have been one of the most exciting scientific and technical innovations of the past few decades. Due to their very high surface to volume ratios, they exhibit properties that can differ dramatically from those for the same material in bulk. This, and their ability to be designed and synthesized with multiple surface functionalities, has seen them used for a myriad of bespoke applications in industry and medicine.
Their medical applications span delivery systems for drugs, proteins and DNA/RNA to diagnostics, targeted cancer treatments, to theranostics. They have been used very successfully for the first-time to deliver mRNA in vaccines against SARS-Cov-2 and have massive potential for revolutionizing vaccine therapies for a wide range of diseases in the future. In industry, nanomaterials are used in hundreds of applications that exploit their unique physicochemical properties, and they are finding use as antimicrobial materials for sanitizing water, clothing, refrigeration etc.
However, commercial and medical applications can outrun the ability of the relevant agencies to regulate nanomaterials. The unusual properties of nanomaterials can potentially generate adverse effects in the workplace, public, and environment and this is particularly an issue for nanomaterials that are introduced to the body, as exposure levels are much higher than the adventitious exposure of workers and the public. Consequently, there is a growing need for nanomaterials that are ‘safe-by-design’ (SbD) that is driving research efforts to achieve that goal. (Guinée et al., 2022; Oksel Karakus et al., 2021)
Why are machine learning models needed?
Experimental assessment of the biological effects of the myriad of commercial and medically applied nanomaterials is infeasible due to time, cost, and resource issues. Computational methods, especially machine learning, are being increasingly used to leverage smaller quantities of experimental data on nanomaterials to predict properties of material not tested or synthesized. Such models can also be invaluable for implementing the SbD paradigm.
Nanomaterials pose particular challenges for computational studies. These materials are much larger than small, discrete molecules like drugs, and their composition is usually not precisely known. Most nanomaterials are distributions of sizes, shapes, surface functionality, and charge. Their surface properties are significantly modulated by the coating of proteins or other macromolecules The so-called nanoparticle corona) that often accumulate on the surface of nanomaterials. (Tomak et al., 2021)
Several key factors determine how effective machine learning models of nanoparticle properties are, and most of these can be problematic for nanomaterials. The first is the quantity, quality, and diversity of data available to train models. There is still a paucity of high-quality data sets for this purpose. The second factor is related to descriptors or features, mathematical entities that capture important physicochemical, provenance, or corona properties of nanomaterials. Given the complexities of nanomaterials mentioned above, converting these structures into meaningful mathematical features is more problematic than for small organic molecules, for example.
The rise of nanosafety consortia
The increasingly large amount of research into modelling the biological (and other) properties of nanoparticles has stimulated the formation of consortia that bring together researchers with complementary skills. In particular, the European Union has supported the development of research networks that aim to provide rapid ways of informing regulators of potential risks of nanomaterials, and create the technologies required to design safety as well as functionality into nanomaterials. This was initiated by EU COST support for a 2011 Maastricht workshop on the use of quantitative structure-activity relationship (QSAR) methods (largely machine learning-based) for predicting the biological effects of nanomaterials. This generated a COST Action, MODENA that generated a network of an impressive core group of researchers in this field, mainly from Europe, but also including Australia and the US.(Winkler et al., 2013) More recently, the EU has provided a large amount of financial support for multiple large nanosafety-related Horizon 2020 projects, the most relevant ones for the theme of this paper being SABYDOMA and NanoSolveIT. These have aimed to generate larger datasets relevant to nanosafety, to explore new ML methods for modelling, and predicting biologically relevant properties and to develop better mathematical descriptions of nanomaterials for training models. (Lynch et al., 2020).
Examples of the application of machine learning to nanosafety
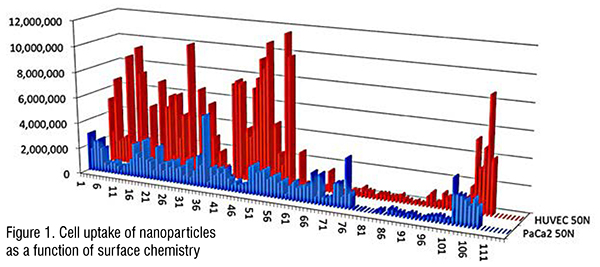
One of the first reports of quantitative modelling of biological properties of nanomaterials was by Puzyn’s group in Gdansk. (Puzyn et al., 2011) They showed that for a series of 17 metal oxide nanoparticles, they could predict cytotoxicity to bacteria using a single, albeit arcane property, the enthalpy of formation of a gaseous cation having the same oxidation state as that in the metal oxide structure. Subsequently, in a collaboration with Harvard University and the Massachusetts General Hospital, we showed that the smooth muscle apoptosis of different types of metal ion oxide nanoparticles could be predicted from their size, core composition, and surface coating. (Epa et al., 2012) This study also showed that, for a set of 109 nanoparticles whose surfaces were functionalized with small organic molecules, the ability to penetrate pancreatic cancer (PaCa2) and human umbilical vein endothelial cell (HUVEC) cells varied markedly depending on the surface chemistry (Figure 1). This was of particular interest in targeting nanoparticles to specific tissues or tumours. Using machine learning models, it was possible to accurately predict the uptake in these cells (within a factor of 2) for both the training set used to create the models and a separate test set used to validate predictions.
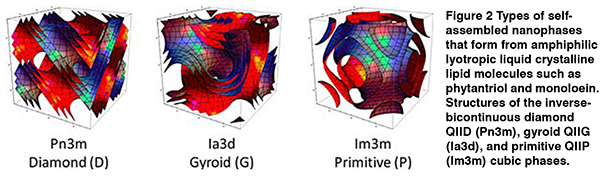
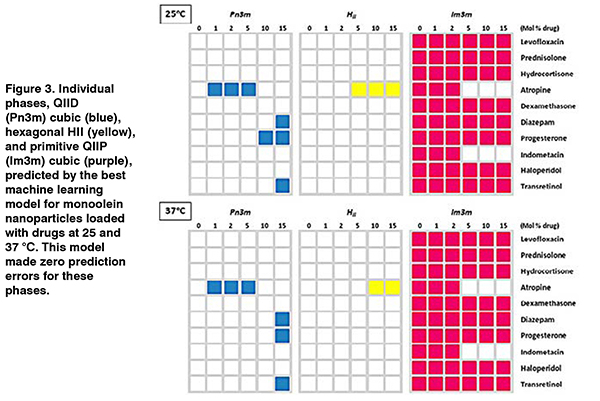
Later work on the nanophase behaviour of amphiphilic, lyotropic liquid crystalline lipid nanomaterials used as drug delivery vehicles (Figure 2) showed that machine learning could predict the existence of multiple, coexisting phases as a function of drug type and concentration, temperature, and time. (Le et al., 2013; Le and Tran, 2019) These models could successfully predict the coexistence of nanophases for new drugs not used to generate the models (Figure 3). As only some of these nanophases are useful for drug delivery, it is important to be able to predict the phase behaviour over time and temperature to ensure drug delivery is optimal and safe.
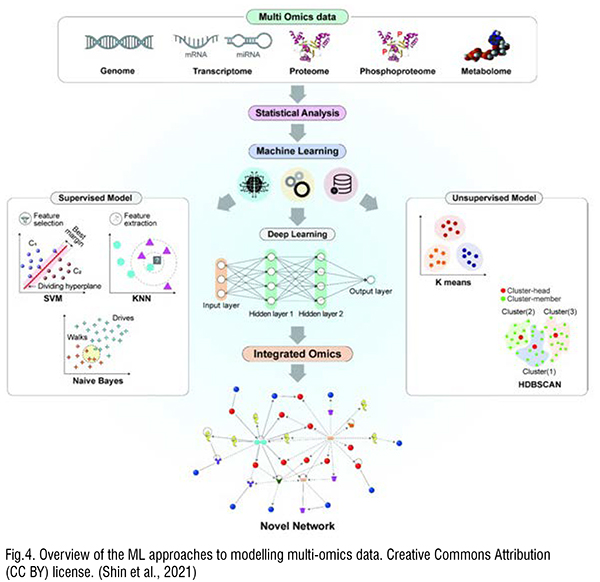
Although most of the work to date has focused on the safety of nanomaterials for commercial applications or generally, there is a growing interest in applying ML methods to ensure the safety of medical nanomaterials. For nanomaterials used in medicine, safety is of course a critical issue as the materials are deliberatively introduced to the body at higher concentrations than that acquired from the environment. Singh et al. recently reviewed how AL and ML invitro and in vivo datasets are used to generate in silico models for nanomedicine. (Singh et al., 2020) They described how physiologically based pharmacokinetic and ADMET (absorption, distribution, metabolism, excretion, and toxicology) in silico methods and dosimetry models can be used to support safety in nanomedicine. Shin and co-workers reviewed the application of ML to multi-omics (transcriptomics, proteomics, metabolomics and phosphoproteomics) data. (Shin et al., 2021) As a complete understanding of the nanoparticle-induced toxicity and other biological changes induced in cells is very challenging, rich omics data combined with ML elucidate mechanistic details, identify beneficial and detrimental nanomaterials properties, and allow prediction of likely biological properties of new medical nanomaterials.
What is needed to generate better models of nanomaterials properties?
Machine learning is a data driven method and the quality and utility of models are strongly dependent on the data and mathematical features used to train them. Recent efforts to synthesize nanoparticles with controlled sizes, shapes, cores, and coatings and adoption of high throughput synthesis and characterization methods should alleviate the current paucity of quality data sets. More extensive use of genomic and proteomic data will also assist in unravelling the mechanisms of biological effects of nanomaterials on organisms and provide additional data sources for modelling. A particularly difficult roadblock is the need for more effective mathematical features (nano-specific descriptors) to describe complex nanomaterials. (Winkler, 2016; 2020; Wyrzykowska et al., 2022) New developments in convolutional neural networks may partially alleviate this problem and lead to enhanced interpretability of models. If more metadata relating to the synthesis and processing of nanomaterials can be captured, then machine learning models can also contribute to the optimisation of bespoke nanomaterials. Other important issues include better data integration at the nanoscale, the need for development of central repositories and databases of nanomaterialsbiodata, robust standards for information storage and exchange, improved nano-ontologies, and better tools for decision support. Ultimately, combined models predicting adverse biological effects and those predicting commercially useful properties will lead to more quantitative, useful, and rational SbD approaches.
References
- Epa, V.C., Burden, F.R., Tassa, C., Weissleder, R., Shaw, S., and Winkler, D.A. (2012). Modeling biological activities of nanoparticles. Nano Lett 12, 5808-5812. 10.1021/nl303144k.
- Guinée, J.B., Heijungs, R., Vijver, M.G., Peijnenburg, W.J.G.M., and Villalba Mendez, G. (2022). The meaning of life … cycles: lessons from and for safe by design studies. Green Chemistry 24, 7787-7800. 10.1039/D2GC02761E.
- Le, T.C., Mulet, X., Burden, F.R., and Winkler, D.A. (2013). Predicting the complex phase behavior of self-assembling drug delivery nanoparticles. Mol Pharm 10, 1368-1377. 10.1021/mp3006402.
- Le, T.C., and Tran, N. (2019). Using Machine Learning To Predict the Self-Assembled Nanostructures of Monoolein and Phytantriol as a Function of Temperature and Fatty Acid Additives for Effective Lipid-Based Delivery Systems. ACS Applied Nano Materials 2, 1637-1647. 10.1021/acsanm.9b00075.
- Lynch, I., Afantitis, A., Exner, T., Himly, M., Lobaskin, V., Doganis, P., Maier, D., Sanabria, N., Papadiamantis, A.G., Rybinska-Fryca, A., et al. (2020). Can an InChI for Nano Address the Need for a Simplified Representation of Complex Nanomaterials across Experimental and Nanoinformatics Studies? Nanomaterials (Basel) 10. 10.3390/nano10122493.
- Oksel Karakus, C., Bilgi, E., and Winkler, D.A. (2021). Biomedical nanomaterials: applications, toxicological concerns, and regulatory needs. Nanotoxicology 15, 331-351. 10.1080/17435390.2020.1860265.
- Puzyn, T., Rasulev, B., Gajewicz, A., Hu, X., Dasari, T.P., Michalkova, A., Hwang, H.M., Toropov, A., Leszczynska, D., and Leszczynski, J. (2011). Using nano-QSAR to predict the cytotoxicity of metal oxide nanoparticles. Nat Nanotechnol 6, 175-178. 10.1038/nnano.2011.10.
- Shin, T.H., Nithiyanandam, S., Lee, D., Kwon, D., Hwang, J.S., Kim, S.G., Jang, Y.E., Basith, S., Park, S., Mo, J.S., and Lee, G. (2021). Analysis of Nanotoxicity with Integrated Omics and Mechanobiology. Nanomaterials 11, 2385. 10.3390/nano11092385.
- Singh, A.V., Ansari, M.H.D., Rosenkranz, D., Maharjan, R.S., Kriegel, F.L., Gandhi, K., Kanase, A., Singh, R., Laux, P., and Luch, A. (2020). Artificial Intelligence and Machine Learning in Computational Nanotoxicology: Unlocking and Empowering Nanomedicine. Advanced Healthcare Materials 9, 1901862. https://doi.org/10.1002/adhm.201901862.
- Tomak, A., Cesmeli, S., Hanoglu, B.D., Winkler, D., and Oksel Karakus, C. (2021). Nanoparticle-protein corona complex: understanding multiple interactions between environmental factors, corona formation, and biological activity. Nanotoxicology 15, 1331-1357. 10.1080/17435390.2022.2025467.
- Winkler, D.A. (2016). Recent advances, and unresolved issues, in the application of computational modelling to the prediction of the biological effects of nanomaterials. Toxicol Appl Pharmacol 299, 96-100. 10.1016/j.taap.2015.12.016. Winkler, D.A. (2020). Role of Artificial Intelligence and Machine Learning in Nanosafety. Small 16, e2001883. 10.1002/smll.202001883.
- Winkler, D.A., Mombelli, E., Pietroiusti, A., Tran, L., Worth, A., Fadeel, B., and McCall, M.J. (2013). Applying quantitative structure-activity relationship approaches to nanotoxicology: current status and future potential. Toxicology 313, 15-23. 10.1016/j.tox.2012.11.005.
- Wyrzykowska, E., Mikolajczyk, A., Lynch, I., Jeliazkova, N., Kochev, N., Sarimveis, H., Doganis, P., Karatzas, P., Afantitis, A., Melagraki, G., et al. (2022). Representing and describing nanomaterials in predictive nanoinformatics. Nat Nanotechnol 17, 924-932. 10.1038/s41565-022-01173-6.













