
Data can be used to analyze genomes, prescribe pharmaceuticals in the pharmaceutical and biotechnology industries, and a variety of other applications. Now that data has become "BIG DATA," analyzing it has become a whole new field of "Analytics and Data Science." The cost of genome structure analysis has decreased from ten million dollars in 2007 to one thousand dollars thanks to analytics. On the basis of big data analysis, a cancer treatment was prescribed. It can be used in a variety of industries and enterprises. There are numerous firms that are solely dependent on analytics, as well as numerous start-ups in the same field. There are ten reasons why pharmaceutical needs Big Data. Drug Discovery, Research and Development and Electronic clinical research, dossier filing and increase business area are just a few of the causes. The pharmaceutical sectors are benefiting from data mining since it is opening up new business options. Big data, data science, tools of data science, and drug discovery, clinical research are some of the terms used in this paper.

The application of data science in the pharmaceutical industry has far-reaching consequences for individuals considering a degree in this discipline. Important statistical data acquired from several sources is regularly used by the pharmaceutical sector. What is the definition of data science? What role does data science play in the pharmaceutical industry and other similar fields? How could a data science degree help you advance in your career? The pharmaceutical industry is a sector that is constantly growing. As more technologically advanced prescription pharmaceuticals become available, the demand for experts who can comprehend and use relevant data and statistics has risen dramatically. What is the role of data science in the pharmaceutical industry? Consider some of the various forms of data that are needed on a regular basis in this profession.
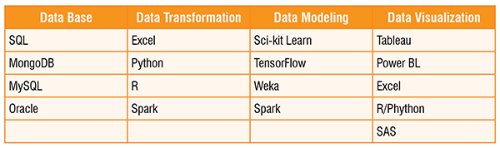
Data is worthless until it is transformed into useful information. Data science is the study of large collections of structured and unstructured data with the goal of revealing hidden patterns to generate valuable insights. The interdisciplinary field of data science includes computer science, statistics, inference, machine learning methods, predictive analysis, and emerging technologies.
A data scientist is responsible for information extraction, manipulation, pre-processing, and prediction from data. To do so, one needs a number of statistical tools and programming languages.
In the pharmaceutical sector, data analysis has become a crucial tool. In fact, pharmaceutical companies are continuously seeking for ways to improve their chances of bringing new treatments to market as fast and inexpensively as possible. Big data applications, such as data governance, data mining, and predictive analysis, are assisting pharmaceutical businesses and researchers in making better judgments about which medications to pursue.
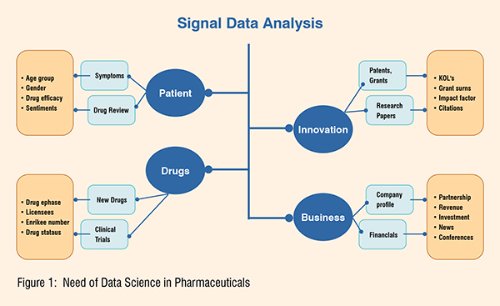
Much of the money spent in big pharma goes toward screening before a drug even reaches the clinical trial stage. This may be a time-consuming and costly process, especially for sick people who are waiting for new treatments to be approved that could help them. Now, data science is being utilised to speed up and hopefully reduce the cost of this previously lengthy procedure. Companies can use predictive analytics to focus on specific goods and ingredients in pharmacological therapies that are most likely to be effective. These selections will be based on a variety of facts acquired to assist them in selecting from the hundreds of options available. In addition, practically all sales and marketing teams rely significantly on targeted analytics to increase sales, improve spending, and improve the bottom line.
Bringing a new medication to market is one of the most dangerous and costly ventures a corporation can undertake. Average cost of a successful drug in the market is roughly $4 billion, but it can reach $11 billion. Given that only 10 to 12 percent of new pharmaceuticalsmake it from the early stages of drug development to the market, the modern pharmaceutical industry has every motive to use data analysis to achieve a competitive advantage. When it comes to avoiding poor outcomes, data analysis can be utilized in clinical drug trials to quickly detect safety or operational signals that need to be addressed in order to avoid major and possibly costly concerns like adverse events and excessive delays. Companies can also discover which groups would work best in trials by combining multiple data sources, such as social media and public health databases.
Data scientists can assist pharma companies in lowering the expenses of clinical trials by allowing them to use:
Provides real-time analysis
Real-time data is now available, which is a feature that will substantially improve trials. This makes it easier to respond to difficulties quickly and provide more precise safety measures for trial participants, all of which leads to improved R&D success rates. Furthermore, data from real-world sources such as health records, insurance claims, and even social media can now be acquired. This gives evidence on how drugs operate in an uncontrolled situation and across a larger demographic to drug makers. This allows them to make changes and improvements to the medications. Data from studies and trials across many diseases is now collected by dedicated teams at major pharmaceutical corporations. Their study of this data allows them to improve the potency and effectiveness of their products while reducing the costs of traditional clinical trials and parallel development projects.
Simplify production plans
A product must be manufactured and distributed after it has been developed. For the highest return on investment, you must understand the suitable targets. Companies can establish a more solid production plan, save labor costs, remove waste, reduce the need for superfluous inventory, and optimize equipment usage with the appropriate data. This simplicity of manufacturing will only grow in the future, both in the healthcare industry and in related businesses. With the pharmaceutical business expected to rise to $1.57 trillion in value, data will play an even bigger role in streamlining production processes.
Prescriptive data analytics with IoT data will be used by 50% of pharmaceutical and biotech industries to optimize their supply chains. Pharmaceutical firms are moving away from traditional procedures and adopting digital transformation and pharmaceutical data analysis on a much larger scale today. As we discussed in our previous article on how to improve customer experience, this move allows them to understand and cater to the demands of both their consumers and stakeholders. By readily validating data, detecting abnormalities, benchmarking processes, and accessing mobile and logistic reports, you can increase your supply chain efficiency with data analytics. Furthermore, data analytics for pharmaceutical development allows for real-time route optimization and better inventory management, freeing up man-hours that would otherwise be spent watching and monitoring business operations. The use of data in the development of pharmaceutical products is extremely advantageous since it aids in the prevention of health problems and the strengthening of the patient care sector.
Predictive models have been increasingly popular in recent years. There is widespread interest in being able to use current data to accurately forecast future trends and results, allowing businesses to meet future demands before they exist. Much of the money spent in big pharma goes toward screening before a drug even reaches the clinical trial stage. This may be a timeconsuming and costly process, especially for sick people who are waiting for new treatments to be approved that could help them. Now, data science is being utilized to speed up and hopefully reduce the cost of this previously lengthy procedure. Companies can use predictive analytics to focus on specific goods and ingredients in pharmacological therapies that are most likely to be effective. These selections will be based on a variety of facts acquired to assist them in selecting from the hundreds of options available.
In previous years, pharmaceutical medication companies' sales and marketing were mostly conducted on foot by paid personnel who would tirelessly visit doctor's offices and medical centres across the country. Because of developments in data science, this action is no longer essential. Drug businesses now do at least 25% of their marketing through digital channels. In addition, practically all sales and marketing teams rely significantly on targeted analytics to increase sales, improve spending, and improve the bottom line. Based on data obtained and evaluated in advance, predictive analytics allows companies to determine which medical experts are more likely to be interested in a given drug. This can lead to the development of highly targeted sales strategies that are more likely to succeed. Additionally, today's drug salespeople are equipped with smart electronic gadgets that provide real-time analytics to assist them in closing the deal. This maximizes their productivity and increases their chances of success.
The pharmaceutical industry can only benefit from the massive amounts of molecular and clinical data housed in proprietary networks (as well as the seemingly limitless amount of consumer data on the Internet) if it can turn it into relevant business intelligence. Data scientists can significantly impact this. In order to create a publicly accessible private cloud where the pharmaceutical industry can safely connect around clinical trial data, several drug researchers and manufacturers are working with data management companies.
References