Over the past two decades the Clinical Supply Chain discipline within the pharmaceutical industry has sought a variety of strategies that help ensure the right medicine gets to the right patient at the right time more effectively and efficiently. One of the first transformative strategies to be developed was drug pooling. Today however, this strategy is still not used pervasively throughout the industry. This article will address the reasons why supplies pooling can be effective and efficient and the barriers to its widespread adoption.

One of the most challenging responsibilities a Clinical Supply Chain Manager has is to determine a reliable supply forecast that stands up to the complexities of the clinical trial design and the various scenarios that can occur during trial conduct. From unpredictable patient recruitment to the inevitable string of protocol amendments, the design of a clinical trial both within the protocol and in operational conduct provides a host of variables that lie outside of supply chain control, and which must be adjusted rapidly from study start-up to study close-out. However, even elements within supply chain control can be subject to unexpected changes such as delays in sourcing, manufacture, packaging, or distribution. While a good supply forecast will plan to mitigate future scenarios of significant risk and impact, no forecast can adequately prepare for all of the unknown combinations of factors without incurring the high costs of supply waste. Thus, the best and most successful supply forecasts are supported by continuous monitoring that serves to evaluate the performance of the forecast over time.
While monitoring their forecast, Supply Chain Managers will often ask questions like have we manufactured enough, have we manufactured too much, or do we have enough in the right place? When the supply is just supporting one trial, this effort to manage supplies is focused and efficient. Most clinical programs however are rarely limited to one protocol, and during Phase III development, it is more likely to be 3-5 trials in some stage of start-up, conduct, or close-out across the globe. It was from the program-level perspective and a need for clinical supplies to be agile, that the supply strategy called Pooling was designed.
Pooling by today’s definition refers to a strategy in which supplies are packaged and labeled in such a way that they can be used across multiple studies, distributed globally, and are adaptable to change. Although the definition of pooling today is focused on this cross-study use of supplies, the concept was born from the evolution of supplies management after IRT technology began to play a prominent role in trial conduct. Not even 20 years ago, clinical supply material labels included pre-printed patient numbers and visit identifiers to properly dispense the right medication according to the randomization of patients to treatment arms. With the advent of centralized randomization through IRT technology, the supplies at the site had to be ready and able to be dispensed to any patient at any visit for which they could be assigned. As a result, patient numbers and visit identifiers were removed from the labels, and the supplies at the depot could be sent to any site and supplies at a site could be dispensed to any patient at any visit which required that drug type. In this way drug was pooled, but within one limitation. That limitation was that the supplies were only pooled within the one study that the supplies were specifically labeled for. It was natural for the clinical supplies industry to then ask could the protocol number be removed so that supplies could be used not only across patients within one study but across patients in multiple studies.
As pooling evolved, the most common implementation has been to have a stock of supplies at a depot ready and able to be dedicated to one study or another up to the point of shipment to a site. In this way, if one study was slow to enroll and another was going faster than expected, the supplies could be easily shifted from one study to another. A less common implementation of pooling is to have a stock of supplies at a site ready and able to be dedicated to one study or another up to the point of dispensation to a patient. This provides maximum flexibility of supplies such that if a site runs multiple trials within the program, the supplies could shift between studies right up to the point of dispensing. This strategy is useful because many clinical programs rely on a common set of sites representing large centers for clinical trial conduct or key opinion leader sites. In either case, depot or site pooling, the major advantages of the strategy stem from delaying the point in time in which a packaged unit is dedicated to a study and thereby maximizing the opportunity for the supplies to be used, increasing supply flexibility to cover various scenarios, and ultimately minimizing supply waste. For the Clinical Supply Manager, pooling also plays a role in simplifying the aggregation of forecasts across multiple studies as they are centrally inventoried within supply systems that control distribution and dispensation, typically an IRT system. Instead of aggregating a forecast from multiple independent study-level reports, pooling strategies consolidate the inventory into centralized reporting for easy visualization during monitoring activities.
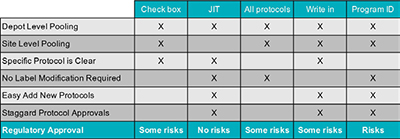
To facilitate drug pooling successfully, a standard packaging configuration and label design is key. The ability to use a common dosing unit in a standard form, like a 30-count bottle labeled for many countries, stakes the foundation of a pooling strategy. While standard packaging designs can also be used in supply strategies that do not employ pooling, it is the labeling strategy that is principally different within a pooled supply. There are multiple ways to adapt a clinical label that supports pooling. These include 1) listing all protocols that the supplies might be used in, 2) providing a list of protocols on the label with an added check box to be filled in when the materials are dedicated to one study over another, 3) Just-In-Time (JIT) labeling at a depot that applies the protocol number on the supplies just before shipping to a site, 4) providing a write-in field on the label to have the study assignment applied by the depot or clinical site, and 5) providing a program identifier alone rather than a specific protocol ID. Each strategy has its benefits and risks. For example, while these labeling strategies can be effective when pooling supplies at a depot level, a JIT labeling strategy cannot be employed if supplies are then also pooled at a site level. Additionally, sites can often feel it is confusing when they see multiple protocols listed on the label especially if they are not involved in all atudies, or when only a program ID is provided. If using checkbox configuration or a write-in field, sites often require additional training on what they must do before dispensing the medication to the patient. In other cases, if the program is rapidly expanding, then a choice to list all protocols, with or without checkboxes, may mean you are continually having to reprint your labels to accommodate additional studies. Moreover, in these cases of having all studies on the label, the approval for use of the supply in any one study is contingent on approval for use in all studies. If there is a delay in any one trial, the supplies will not be released for use in the other trials. Finally, each labeling strategy has various levels of regulatory acceptance as shown in the table below. As depot-level pooling is most often the strategy of choice, and JIT labeling is well accepted by the authorities, applying the protocol number at the point of distribution is a commonly employed strategy when pooling is adopted. See the table below for a side-by-side comparison of these different strategies.
The second key to facilitate pooling is to employ a technology such as IRT that can assign the supplies to a study or a patient. GXP regulations require a method that can trace any one dispensable unit through the course of events from manufacture to site, site to patient, and finally from return to destruction. These requirements impose a study-level accounting of supplies within the study and are the point at which the supply dedicated to the study must be documented and traced. IRT technology is a perfect choice for the job since it already traces the supplies from depot to patient and often, to the point of destruction as well. While the early step to pool supplies across patients is commonly employed, and IRT technology supplies a means to do it, taking the additional step to pool drugs across studies at either the depot level or the site level is rarely used within the industry. Instead, most supplies are labeled for each specific trial regardless of how many studies use the same packaging design.
There are many factors that have prevented the more widespread use of pooling within clinical programs. One of these factors is the readiness of many regulatory agencies to embrace the strategy and adapt their laws to enable it more easily. In countries that employ import license requirements, for example, supplies must be dedicated to a specific study to gain an import license, and thus drug pooling across studies is inhibited within those countries. In the EU, the early Clinical Trial Directive provided language that supported the use of IRT to indicate labeling information and thus permitted pooling. Specifically, Article 13 Section 26 stated that “information should be included on labels, unless its absence can be justified, e.g., use of a centralized electronic randomisation system” and listed the trial reference code as one such piece of information that could be “given elsewhere” (Directive 2001/20/EC 2001). However, as it was a directive and not a law, there was variability across countries in supporting such strategies, and thus few readily approved label templates that did not specifically show the protocol number on them. As a result, and coupled with the fact that most companies are risk-averse when it comes to challenging regulatory pushback, few companies chose to adopt pooling strategies. There was some hope when the EU Clinical Trial Regulation was drafted to resolve these country-by-country differences, but the regulation, when it was first published, only reinforced a preference toward protocol-specific labeling.

Another factor that has been a barrier to the widespread adoption of pooling is the growing trend of clinical supply organizations adopting ERP technology systems within their clinical supply chain. Most of these systems were designed to support commercial operations where there is one label per package and a dedicated distribution stream. As pooling requires the supplies to exist in a partially undefined state, which ERP systems do not easily support, a pooling strategy was difficult to implement when these systems were in use. Additionally, while many IRT systems took steps to incorporate pooling methodologies when the idea was first developed, the lack of interest has failed to inspire further innovation which might work to promote its use. While several IRT solutions can support drug pooling, especially at the depot level, it is often a specific customization of the system rather than a standard in configurable templates. Thus, the development timelines for IRT systems with pooling can be subject to additional build time, which most protocol teams are not in favor of.
As with any strategy, there are tradeoffs between benefits and risks. Pooling, nonetheless, has been well proven to reduce the overall cost of the clinical supply chain and mitigate the risk of stock out when multiple protocols are ongoing in a clinical program. The keys to the successful implementation of pooling strategies include a standard packaging design, a fit-for-purpose labeling strategy, an IRT system that has robust pooling functionality, thoughtful planning for what countries will best support a pooling strategy, and most of all a strong partnership between clinical operations, the CRO, the supply chain, and the supporting regulatory group such that there is alignment that the benefits of pooling far outweigh any risks. For large phase III programs that are managing several trials at once, pooling works to ensure that the right investigational supplies are in the right place and the right time, and in the right amount and there is no greater achievement for a Clinical Supplies Manager.