High affinity of a compound for red blood cells can lead to its sequestration in the cells but this property of certain drugs may be overlooked in the process of drug development. This article will illustrate that measuring drug concentration in both compartments - plasma and red blood cells is required for accurate pharmacokinetic assessment of drugs.
The blood-to-plasma ratio (B/P ratio or Rb) is an important parameter in pharmacokinetics (PK) that reflects the distribution of a drug between red blood cells (RBCs) and plasma. It is defined as the ratio of the concentration of a drug in whole blood (CB) to the concentration of the drug in plasma (CP). Drugs that have a B/P ratio greater than 1 tend to accumulate in RBCs. Approximately 55% of the whole blood is composed of plasma and the remaining 45% consists of various blood cells including RBCs, white blood cells, and platelets. When a drug reaches the bloodstream, it can either reside in plasma or it can partition into blood cells. Among the blood cells, RBCs take up approximately 99% of cellular space and therefore RBCs are the only major compartment in blood to be accounted for apart from plasma for PK assessment. However, as a standard practice, drug concentration is typically only measured in plasma. For a drug with a high B/P ratio, PK parameters derived solely from the drug concentration in plasma may be misleading because the portion of the drug that is sequestered in RBCs is not accounted for. The intrinsic clearance of drugs with high B/P ratio, which has been estimated only based on drug concentrations in plasma, may significantly overestimate the volume of distribution and overall blood clearance. This may also lead to calculated clearance values exceeding the hepatic blood flow. For drugs with a high affinity for RBCs, the drug concentration in the whole blood will be a more accurate measurement for PK studies. In reality, drugs with a high B/P ratio may have a longer half-life due to slow release from blood cells, affecting dosing regimens and the time to achieve steady-state concentrations. Hence, it is of critical importance to decipher the distribution of a drug in plasma vs. RBCs for appropriate estimation of a drug’s PK.
Drugs can partition into RBCs in several different ways. Lipophilic organic compounds will dissolve in the lipid bilayer of the membrane and enter the RBCs. Small hydrophilic compounds can enter by diffusion. Transporters or carrier proteins can also play a role in a drug’s transport into RBCs. As a general rule, nonionized and basic drugs tend to distribute evenly between plasma and RBCs with Rb values close to 1, whereas zwitterionic and acidic drugs tend to be excluded from RBCs with Rb values of roughly 0.55 (1‒hematocrit). The B/P ratio can vary greatly among different drugs depending on their physicochemical properties, binding affinity of a drug to plasma proteins, and the health status of the individual.
Understanding the drug’s partitioning in RBCs, kinetics of reversibility of such partitioning, and any species-specific differences early in the process of drug development are very useful in determining whether the PK should be estimated using the B/P ratio, selecting the right matrix for routine PK sampling if needed, confirming toxicology species selection, dosing interval determination, and so on. An additional aspect of the B/P ratio determination is its inclusion in the calculations recommended by regulatory agencies that help with the in vitro to in vivo prediction of potential clinical drug-drug interactions (DDI) among co-medications.
There are several examples of drugs that are approved for use despite their high B/P ratios that are discussed in more detail below. Certain drugs, in fact, have leveraged RBCs as a vascular carrier for targeted drug delivery. Overall, the B/P ratio turns out be an important parameter influencing PK behavior, efficacy, toxicity, and DDI potential, unintentionally or intentionally.
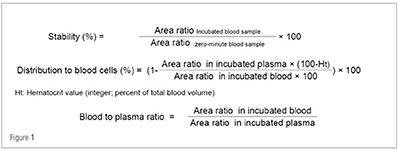
The extent of distribution of a drug into RBCs can be determined in vitro by incubating the drug with fresh whole blood. Prior to the procedure, whole blood is collected in tubes with the anticoagulant K2EDTA and an aliquot is pre-warmed in a water bath at 37 °C. The test samples are incubated with the drug candidate at various concentrations for the designated time period (e.g., 60 minutes). At the end of the incubation, an aliquot is collected and transferred to a tube containing water and immediately added to the precipitating organic solution (e.g. 70:30 v/v acetonitrile:methanol) containing the appropriate internal standard. The remaining whole blood incubation is centrifuged to separate the plasma. After centrifugation, an aliquot of plasma is transferred to a tube containing water and added to the precipitating organic solution (e.g. 70:30 v/v acetonitrile:methanol) containing the appropriate internal standard as in the previous step. All blood and plasma samples are then centrifuged to separate the precipitated protein and the supernatants are analyzed by LC-MS/MS to quantitate analyte/internal standard area ratios. Simultaneously, zero-time blood and plasma samples are subjected to the same procedure as the test samples. In addition, incubations with a high affinity control like chloroquine and a low affinity control like metoprolol are also included in the assay. Hematocrit is also measured by adding an aliquot of whole blood into a capillary tube and centrifuging it. Upon the collection of LC-MS/MS based concentration data, the drug’s metabolic stability and B/P ratio are calculated as follows:
B/P ratio can be measured using this procedure in various animal species such as mouse, rat, dog, monkey, etc., in addition to human. The choice of the species can be determined based on the potential nonclinical toxicology study species and would be beneficial to determine any species-specific differences in PK owing to Rb differences. Typically, the assay is conducted using a range of concentrations of the candidate drug (at least three concentrations) to determine potential saturation phenomena. Stability assessment over the incubation time determines whether the drug candidate is metabolized or degraded enzymatically or non-enzymatically in whole blood. Generally, the extent of cellular drug partitioning in blood observed in vitro is similar to in vivo. However, there may be instances with drugs such as metformin where the in vivo system may be very different from the values obtained in vitro when repartitioning from blood cells is far slower than clearance of drug in plasma.
Understanding the drug’s partitioning into RBCs is also beneficial to determine the appropriate matrix for PK i.e., whole blood vs. plasma or inclusion of stabilizers such as inhibitors of hydrolases or glutathione to scavenge free radicals in the plasma. RBC partitioning may vary for certain drugs depending on the temperature. Reports have indicated that the RBC partitioning is not affected if the assay is run at 4°C for most drugs. However, for drugs that have high affinity to RBC components such as carbonic anhydrase, the 4°C approach may underestimate Rb values and alternative approaches should be used. In these situations, using appropriate inhibitors to minimize instability issues can be considered. RBC partitioning may also vary as a result of pH-dependent binding of a drug to plasma proteins and/or RBCs. In these situations, and other instances where the limit of quantitation is not low enough to quantitate a drug in plasma due to the majority of the drug being in RBCs, whole blood may be a rational choice of matrix for PK quantitation.
Rb can be applied to convert drug PK parameters in plasma to the respective parameters in whole blood and to develop in vitro–in vivo correlations for DDI determination. The equation Rb = CB/CP can be rearranged as CB = CP x Rb in order to determine the whole blood concentration of a drug. In the static model used to predict clinical potential of a drug for interactions with hepatic uptake transporters such as organic anion transporting peptide (OATP) 1B1, OATP1B3, and organic cation transporter 1, the relevant in vivo concentration is the unbound maximum hepatic inlet concentration (Iin,max,u) of an orally administered test drug. The three regulatory agencies, the United States Food and Drug Administration, European Medicines Agency, and Japan’s Pharmaceuticals and Medical Devices Agency, define the Iin,max,u with some nuances, however, with the use of Rb, all these values of unbound maximum hepatic inlet concentration are the same.
There are several drugs reported in the literature that exhibit Rb greater than 1, indicating higher distribution to RBCs compared to plasma. They include sirolimus, everolimus, rivaroxaban, dorzolamide, metformin, gemcitabine, indapamide, acetazolamide, methazolamide, doxycycline, topiramate, doxorubicin, chlorthalidone, phenprobamate, romifidine, cyclosporine, quinidine, just to list a few.
Understanding the mechanistic aspects such as linearity of RBC partitioning and kinetics of reversibility of RBC partitioning is important before using Rb to interconvert values of drug concentration in blood and plasma and/or clearance from blood and plasma. The following examples illustrate these differences.
Tacrolimus, an immunosuppressive, is one of the drugs that exhibits a high B/P ratio. In liver transplant patients, Rb may range from 13 to 114. There is significant variation in the plasma vs. whole blood clearance (CL) and volume of distribution (Vd). CL and Vd were much higher based on plasma concentrations (CL=1.7 L/h/kg; Vd= 30 L/kg) compared to whole blood (CL=54 ml/h/kg; Vd= 0.9 L/kg). Binding to RBCs affects the disposition of tacrolimus, and plasma concentrations are indirectly and inversely related to it. In this case, nonlinear binding of tacrolimus to RBCs was found to be a major source of interpatient variation in the disposition of the drug. Tacrolimus has a narrow therapeutic index and therefore, to optimize dosing, therapeutic drug monitoring is required on the basis of systemic exposure that is typically measured in whole blood.
Some diuretic drugs such as chlorthalidone, dorzolamide, and methazolamide exhibit a high Rb. These drugs bind with high affinity to carbonic anhydrase, an abundant enzyme in RBCs. These drugs have Rb values ranging from 30 to 240, but they do not move freely between the RBCs and plasma. Hence, for these drugs—whose movement out of RBCs is restricted—it is their concentration in plasma that is relevant to their potential to cause drug interactions.1
High Rb is beneficial for drugs that have RBCs as their targeted site of action, e.g., anti-malarial drugs. Accumulation of the drug in RBCs increases its half-life and consequently increases the efficacy of the drug. Anti-malarial drugs such as chloroquine, amodiaquine, mefloquine, piperaquine, proguanil, quinine have Rb greater than1.
Another interesting upcoming advance in drug development is RBC-based drug carriers. RBCs represent attractive natural carriers that can be loaded with a drug of interest either in vitro or ex vivo. Recent studies showed that RBC-based drug carriers indeed may feature unique PK and biodistribution characteristics favorably changing the benefit/risk ratio of some cargo agents.
RBCs occupy approximately 45% of the blood space and distribution of a drug to RBCs impacts the overall PK of a drug. Therefore, it is imperative that Rb is measured in routine PK analysis. Rb and an understanding of the extent and reversibility of RBC partitioning of a drug are also necessary to delve effectively into intricate PK-based decision making.
References
1. Hinderling PH. Red blood cells: a neglected compartment in pharmacokinetics and pharmacodynamics; Pharmacol Rev 49:279-295, 1997
2. Parkinson A. Regulatory Recommendations for Calculating the Unbound Maximum Hepatic Inlet Concentration: A Complicated Story with a Surprising and Happy Ending; Drug Metab Dispos 47:779–784, 2019
3. Xie F, Ke AB, Bowers GD, Zamek-Gliszczynski MJ. Metformin's Intrinsic Blood-to-Plasma Partition Ratio (B/P): Reconciling the Perceived High In Vivo B/P > 10 with the In Vitro Equilibrium Value of Unity. J Pharmacol Exp Ther 354:225-229, 2015.
4. Novak JJ, Burchett W, Di L. Effects of low temperature on blood-to-plasma ratio measurement. Biopharm Drug Dispos 42:234-241, 2021
5. Dash RP, Veeravalli V, Thomas JA, Rosenfeld C, Mehta N, Srinivas NR. Whole blood or plasma: what is the ideal matrix for pharmacokinetic-driven drug candidate selection? Future Med Chem 13:157-171, 2021
6. Shank RP, Doose DR, Streeter AJ, Bialer M. Plasma and whole blood pharmacokinetics of topiramate: the role of carbonic anhydrase. Epilepsy Res 63:103-112, 2005
7. Jusko WJ, Piekoszewski W, Klintmalm GB, Shaefer MS, Hebert MF, Piergies AA, Lee CC, Schechter P, Mekki QA. Pharmacokinetics of tacrolimus in liver transplant patients. Clin Pharmacol Ther 57:281-290, 1995
8. Pornputtapong N, Suriyapakorn B, Satayamapakorn A, Larpadisorn K, Janviriyakul P, Khemawoot P. In silico analysis for factors affecting anti-malarial penetration into red blood cells. Malar J 23;19:215, 2020
9. Glassman PM, Villa CH, Ukidve A, Zhao Z, Smith P, Mitragotri S, Russell AJ, Brenner JS, Muzykantov VR. Vascular Drug Delivery Using Carrier Red Blood Cells: Focus on RBC Surface Loading and Pharmacokinetics. Pharmaceutics 12:440, 2020