
Dementia is the first sign of neuronal impairment related to Alzheimer’s disease (AD). The neurodegeneration initially appears in the hippocampus and cortex which comprises memory developing area of the brain and then spreads to additional areas of the brain causing significant shrinkage of the brain. Due to this abnormality accumulation of amyloid β plaques and tau tangles occurs consequently leading to disruption of the functioning of healthy neurons and loss of synaptic connections. Donepezil, memantine, rivastigmine, galantamine, and their combinations are primarily used for the treatment of symptoms of Alzheimer’s. The therapeutic action of these drugs is primarily based on the principle of regulation of neurotransmitter levels and establishing neuronal synaptic connections for the efficient functioning of the brain. However, these drugs are effective for some people for a limited duration because of the drug’s inability to target underlying disease pathogenesis and cure it permanently. Despite these conventional therapies and nontherapeutic approaches such as physical activity, diet, and cognitive training, several clinical trials are ongoing on for discovering precise interventions for getting efficient treatment strategies. Stem cell therapy is a unique and advanced approach which have improved characteristics of self-renewal, proliferation, differentiation, and recombination and can be beneficial for the treatment of Alzheimer’s by regulating the neuronal damage along with their regeneration. Some research organizations like Alzheimer’s Society reinforce the advancement of stem cell therapy to find out an efficient therapeutic intervention for the permanent cure of Alzheimer’s disease. Extracted adult stem cells or embryonic stem cells are converted into induced pluripotent stem cells (iPSCs)that revert into stem cell under laboratory conditions and facilitate regeneration of neuronal cells. A combination of nanotechnology and stem cell therapy can miraculously treat neurodegenerative disorders, may be permanently. Nanocarriers co-embedded with stem cells and drug regulate the cellular microenvironment for regeneration of neurons, re-establishing neuronal synaptic connections, also improve the efficiency of cell and delivery of drug (Donepezil, memantine, rivastigmine, galantamine) to the brain, and also enhance the survival of stem cell transplants. Drug-loaded nanocarrier embedded with stem cells can significantly ameliorate learning and memory functions in AD, replenish basal neuronal activity, and help in the recovery of hippocampal synaptic interconnection for memory development.
1. Introduction
Alzheimer’s disease (AD)is a neurological disorder that is responsible for brain atrophy and neuronal degeneration resulting in a decline in thinking ability and behaviour. The progressive neuro-degeneration leads to memory loss or dementia which makes it difficult to carry out day-to-day tasks and it also has an impact on the socializing ability of the individual[1].Treatment of Alzheimer's is complex due to the involvement of multiple pathophysiological pathways[2].In Alzheimer’s lower levels of acetylcholine are found in brain that is important for learning and memory. Use of cholinesterase inhibitors such as donepezil, galantamine, and rivastigmine reduce the breakdown of acetylcholine which helps to stabilize some of symptoms of Alzheimer’s temporarily[3].Similarly, memantine (Ebixa®) is an NMDA (N-methyl-D-aspartate) receptor antagonist that protects brain cells by blocking the effect of expression of glutamate in AD[4].Tremendous effort is being made throughout the world to develop effective therapies for the management of AD including adopting measures to slow down the loss of memory. Besides these conventional therapies, focus is shifting towards the use of biomarkers, and other pharmaco therapies for the therapeutic management of AD. Of these, use of stem cells is also gaining ample attention as it offers corrective measures at the cellular level[5].The stem cell action is generally mediated by neuroprotective and anti-amyloidogenesis mechanism that can be beneficial for neuronal modulation and regeneration in AD [6]. The therapeutic benefits of stem cells can be enhanced manifold by the application of nanotechnology and can be used for effective management of brain health in the case of AD[7,8].
2. Pathophysiology of Alzheimer’s
Pathophysiology of Alzheimer's disease involves several factors such as cholinergic insufficiency, β-amyloidplaque accumulation, formation of tau tangles, mitochondrial dysfunction, and oxidative stress resulting in neurodegeneration (Figure 1)[9]. Neuronal loss is particularly seen in the hippocampus, amygdale , and the cortical association areas and subcortical nuclei. The accumulation of β-amyloid/tau plaque tangles correlates with cognitive decline and brain atrophy, along with hippocampal atrophy which is associated with inflammation. Amyloid precursor protein split into amyloid β peptides due to activity of α-secretase, β-secretase, and γ-secretase[10,11]. The imbalance between the generation of amyloid β and their clearance causes toxicity of protofibrils, tangles, and plaques[12].
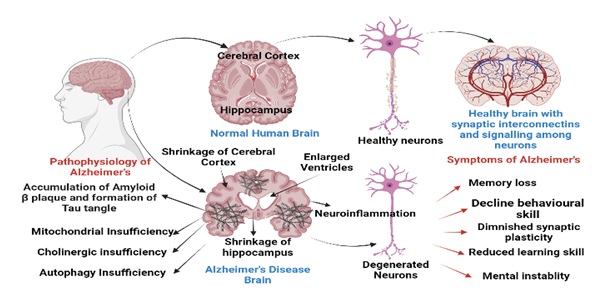
Figure 1: Pathophysiology, anatomy and symptoms of brain inAlzheimer’s disease
3. Stem cell therapy
Neuronal cell impairments are generally irreversible processes with limited possibility of regeneration of neurons. Therefore, the options for the treatment of neurodegenerative diseases are limited. Stem cell therapy can provide an effective therapeutic intervention for the management of neurological impairments and atrophic conditions like those exist in AD[13,14].These potent cells are extracted from bone marrow, adipose tissue, etc. The somatic stem cells are generally harvested from humans and animals as shown in figure 2.Studies have shown the potential of mesenchymal stem cell (MSC)-based treatment in animal models of neurological disorder. Further, some clinical trialshave been conducted on MSCs administered viaintravenous (IV), intra-arterial (IA) routes, or intracerebral (IC) routes and have shown markedly lower brain edema and lesion[15].In animal models, application of stem cell therapy demonstrated improved functional recovery as a result of increased axonal density leading to secretion of neuronal development factors which promoteneurogenesis and recovery of ischemic lesions[16]. Amyloid β accumulation accelerates neuronal loss and cognitive dysfunction. Preclinical studies conducted using stem cells in AD animal models have reported improvement in cognitive function by reducing amyloid β plaques and facilitated autophagy. In addition, treatment with polymeric nanocarriers grafted with MSCs resulted in increased neurogenesis in the hippocampus by enhanced cell proliferation and neuronal differentiation[17,18].Moreover, neuronal regeneration enhances the upregulation of neurotrophic factors such as brain-derived neurotrophic factor (BDNF), vascular endothelial growth factor (VEGF), and nerve growth factor (NGF) in the AD brain. Some researchers have suggested that MSCs potentiate levels of acetylcholine and help in the functional improvement of hippocampal neurons.Moreover, administration of MSCs help in reduction of tumor necrosis factor (TNF-α), and interleukin (IL-6) in microglial, mice cortex to improve neuron survival in AD [19,20].
Pluripotent stem cells have the ability to proliferate and differentiate into cells that are used in regenerative medicine. Pluripotent stem cells are mainly categorized into two classes i.e., somatic stem cells and embryonic stem cells; these cells can differentiate into various kind of cells such as adipose tissue, bone, cartilage, liver, and neural tissue with efficient proliferating ability [21]. Initially, pluripotent cells differentiate into one of three germ layers (ectoderm, mesoderm, and endoderm) and thereafter convert into multipotent stem cells, capable of differentiating into various organs of the human body. The pluripotent cells are treated to form induced pluripotent stem cells (iPSC) or embryonic pluripotent stem cells which can be further used in regenerative therapy in degenerative diseases[18].
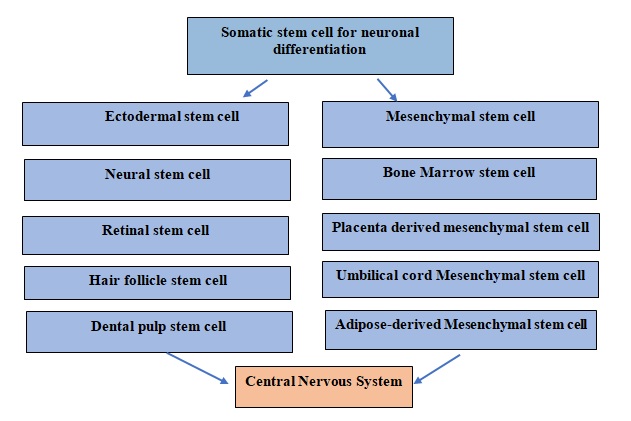
Figure 2: Somatic stem cells used for the neuronal regeneration in central nervous system
4. Principle mechanism of stem cell therapy for the treatment of Alzheimer’s disease
MSCs when administered intra cerebrally release paracrine factors such as brain-derived neurotrophic factor (BDNF), nerve growth factor (NGF), and vascular endothelial growth factor (VEGF) in brain that help in the regeneration of neurons along with development of synaptic connections as shown in figure 3. The regeneration of synaptic connections results in improvement of memory function and learning skills in AD[22]. The neuronal structure consists of severalsubtypes such asdopaminergic neurons, glutamatergic neurons, GABAergic neurons, and cholinergic neurons[23].The neurons in AD have reduced expression of β-secretase and thus cleavage of Amyloid precursor protein(APP) along with lower production of amyloidβ that results in neuronal degradation[24].AD also occurs due to elevation inβ-secretase cleavage of APP induced by glutamatergic neurons that results in accumulation of Aβ and tau phosphorylation[25]. Moreover, microglial dysfunction contributes to the development of AD that contributes in the abnormal clearance of degenerated neurons, synaptic loss, and neuroinflammation in the brain. Therefore, stem cell therapy is helpful in connecting bridge between abnormal production and clearance of amyloid β and improving synaptic plasticity.
Figure 3: Mechanism by which stem cells help in neurogenesis in Alzheimer’s disease.
5. Application of Polymeric Nanocarriers in stem cell therapy
The therapeutic delivery of stem cells even with modest efficacy into the brain is limited due to presence of BBB. Nanocarriers are a versatile platform which can be used for the delivery of stem cells in brain. Two main pathways that facilitate the penetration of drugs, stem cells, proteins, and peptides across BBB are passive transport and active transport. Moreover, functionalization of nanocarriers facilitate delivery of stem cells across BBB and can be modulated for efficient targeting of molecular mechanisms of disease. The use of polymeric nanocarriers for stem cell therapy in neurodegenerative disorders has been elaborately studied and found to be beneficial[26]. Nanoparticle constitutes of PLGA [poly(lactic-co-glycolic acid)] and PLLA [poly(d,l-lactide)] in the particle size range of 50 and 100 nm grafted with stem cells using Induced pluripotent stem cells (iPSCs ) technology have been prepared and studied[27]. These grafted constitutes were cultured and proliferated in human brain microvascular endothelial cells (hBMECs)resulting in the improvement of brain microenvironment due to selective delivery of stem cells across BBB from the core of PLGA and PLLA nanoparticles thereby causing neuronal integration and regeneration. The efficacy of iPSC-based model is by virtue of its efficiency to interact at molecular level and this correlates aptly to the nanoparticles in terms of their size, and composition [27,28].
6. Polymeric nanocarriers grafted with Bone Marrow Stem Cells for the treatment of Alzheimer’s disease
The nanocarriers can be prepared using either natural polymers (gelatin orchitosan) or synthetic ( poly-Ɛ-caprolactone); biodegradable polymers (poly (lactic-co-glycolic acid (PLGA)) ornon-biodegradable polymers (polyurethane).The biodegradability, and biocompatibility of polymeric nanoparticles facilitate delivery of molecules, proteins, nucleic acid, peptides, and stem cellsacross the brain[29]. Structural modification like surface engineering with ligands provide targeting specificity across BBB.
Polymeric nanocarriers grafted with bone marrow stem cells (BMSCs)have been studied for the treatment of AD [27].Eight-month-old mice BMSCs were extracted from endothelial cell line derived from mice and these primary single cells were suspended in a medium and then isolated and cultured at 37°C, 5% CO2, and 80% relative humidity. The cultured cells were amplified at 7–10-day interval into third generation of pluripotent stem cells. The third-generation cultures were further digested with 0.05% trypsin-EDTA before transplantation[30]. The BMSCs were incorporated into polymeric nanoparticle by incubation of stem cells and nanoparticles simultaneously in sterile media. Anesthetized mice were shaved at scalp area and a stereotaxic apparatus was fixed on mice and Hamilton microsyringe was inserted under the dura, and polymeric nanoparticle incorporated with bone marrow stem cells were administered into the brain. Following the treatment procedure behavioural changes were noticed in all mice observed by the Morris water maze test which is used to evaluate learning and memory. The BMSCs group mice showed significantly higher escape latency, and % of distance travelled in the target quadrant, mean speed, as well as crossings of the target platform, was noticed which suggests alleviation in learning and memory deficits in AD mice after BMSCs transplantation. The effect of BMSCs was further assessed on Aβ pathology in the AD brain. These results showed that BMSCs significantly reduced amyloid β1–40 and amyloid β1–42 in the cortex and hippocampus region of AD mice. Quantification of Aβ plaque number, area, and intensity indicated reduction of plaquearea, and intensity in the cortex and hippocampus region of the brain in BMSC streated mice. Neuronal regeneration was also noticed in MRI imaging and histology studies indicating the proficiency of BMSCs to proliferate neurons and re-establish synaptic connection in AD.
7. Challenges and future perspectives
Use of combinational approach of nanotechnology along with stem cell technology might pose some challenges to researchers with respect to optimization and tuning of NPs and their impact on cell differentiation. Apart from these, few concerns regarding preparation, characterization of3-D nanostructures for the purpose of tissue engineering, and substitution of drugs with stem cells exist besides carrying out intense investigation to prove their safety and efficacy for their use in regenerative therapy [31]. All of the drugs currently being used work by regulating neurotransmitters, the chemicals that transmit messages between neurons, however, these drugs provide symptomatic relief with no effect on the root cause of the disease.
8. Conclusion
The increasing burden of AD and its treatment is becoming a global challenge for old-age people as they suffer from severe dementia and behavioural issues. Currently marketed drugs are used for the symptomatic management of AD via regulating levels of neurotransmitter in brain. Apart from the conventional therapeutics, there is a need for such therapies which can be used for treatment of AD. Unfortunately, in AD there is loss of neurogenes is due to inactivation of endogenous neuronal stem cells (NSCs). NSCs lose their potential to produce enough cells along with further integration into neural network that is required to repair neurological damage. iPSC technology is an emerging technique which can be used in the treatment of AD by promoting neurogenesis as a result of the stimulatory action of transplanted stem cells. The transport of stem cell across BBB limits the translation of the technique to the clinics which can addressed by the use of nanocarriers. Preclinical studies (in AD models) have provided significantly optimistic results after administration of polymeric nanoparticles grafted with BMSCs showing decrease in Aβ accumulation, upregulation of acetylcholine levels, and higher neuronal survival and regeneration. Moreover, BMSCs integrate into degenerated neurons leading to enhancement in neurogenesis in the hippocampus thereby stabilizing synapses which help in improvement in memory and behaviour. Additionally, BMSCs improve angiogenesis by stimulating paracrine factors like vascular endothelial growth factor (VEGF) and neuron growth factor(NGF) and brain derived neurotrophic factor (BDNF) which influence the capillary blood flow and neurogenesis in the CNS. Therefore, transplantation of exogenous BMSCs stimulates endogenous neural progenitors’ cells that protect CNS against damage from neurodegeneration. Therefore, there is a dire need of additional studies for exploring the therapeutic potential, safety, limitations and toxicity of the stem cell therapy for the treatment of AD rather than just the symptomatic management which is the current mode of taking care of AD.
9. Acronym
[poly(d,l-lactide) Alzheimer’s disease
PLLA, 5 (AD), 1
[poly(lactic-co-glycolic acid)] Amyloid precursor protein
PLGA, 5 (APP), 5
Bone marrow stem cells Mesenchymal Stem Cells
(BMSCs), 6 (MSCs), 1
Brain-derived neurotrophic factor nerve growth factor
(BDNF), 3 (NGF), 3
human brain microvascular endothelial cells N-methyl-D-aspartate
(hBMECs), 6 NMDA, 2
induced pluripotent stem cells Tumor necrosis factor
(iPSCs), 1 (TNF-α), 4
Interleukin vascular endothelial growth factor
(IL-6), 4 (VEGF), 3
10. References
[1] Vickers JC, Dickson TC, A. Adlard P, Saunders HL, King CE, McCormack G. The cause of neuronal degeneration in Alzheimer’s disease. Prog Neurobiol 2000;60:139–65. https://doi.org/10.1016/S0301-0082(99)00023-4.
[2] Dementia n.d. https://www.who.int/news-room/fact-sheets/detail/dementia (accessed July 29, 2022).
[3] Grossberg GT. Cholinesterase Inhibitors for the Treatment of Alzheimer’s Disease:: Getting On and Staying On. Curr Ther Res Clin Exp 2003;64:216. https://doi.org/10.1016/S0011-393X(03)00059-6.
[4] Olivares D, Deshpande VK, Shi Y, Lahiri DK, Greig NH, Rogers JT, et al. N-Methyl D-Aspartate (NMDA) Receptor Antagonists and Memantine Treatment for Alzheimer’s Disease, Vascular Dementia and Parkinson’s Disease. Curr Alzheimer Res 2012;9:746. https://doi.org/10.2174/156720512801322564.
[5] Fan X, Sun D, Tang X, Cai Y, Yin ZQ, Xu H. Stem-cell challenges in the treatment of Alzheimer’s disease: a long way from bench to bedside. Med Res Rev 2014;34:957–78. https://doi.org/10.1002/MED.21309.
[6] Liu XY, Yang LP, Zhao L. Stem cell therapy for Alzheimer’s disease. World J Stem Cells 2020;12:787. https://doi.org/10.4252/WJSC.V12.I8.787.
[7] Khan NH, Mir M, Ngowi EE, Zafar U, Khakwani MMAK, Khattak S, et al. Nanomedicine: A Promising Way to Manage Alzheimer’s Disease. Front Bioeng Biotechnol 2021;9:236. https://doi.org/10.3389/FBIOE.2021.630055/BIBTEX.
[8] Vissers C, Ming G-L, Song H. Nanoparticle technology and stem cell therapy team up against neurodegenerative disorders. Adv Drug Deliv Rev 2019. https://doi.org/10.1016/j.addr.2019.02.007.
[9] Rajmohan R, Reddy PH. Amyloid Beta and Phosphorylated Tau Accumulations Cause Abnormalities at Synapses of Alzheimer’s disease Neurons. J Alzheimers Dis 2017;57:975. https://doi.org/10.3233/JAD-160612.
[10] Serrano-Pozo A, Frosch MP, Masliah E, Hyman BT. Neuropathological Alterations in Alzheimer Disease. Cold Spring Harb Perspect Med 2011;1. https://doi.org/10.1101/CSHPERSPECT.A006189.
[11] Dam D Van, Vermeiren Y, Dekker AD, Naudé PJW, Deyn PP De. Neuropsychiatric Disturbances in Alzheimer’s Disease: What Have We Learned from Neuropathological Studies? Curr Alzheimer Res 2016;13:1145. https://doi.org/10.2174/1567205013666160502123607.
[12] Hampel H, Hardy J, Blennow K, Chen C, Perry G, Kim SH, et al. The Amyloid-β Pathway in Alzheimer’s Disease. Mol Psychiatry 2021 2610 2021;26:5481–503. https://doi.org/10.1038/s41380-021-01249-0.
[13] Hassan AU, Hassan G, Rasool Z. Role of Stem Cells in Treatment of Neurological Disorder. Int J Health Sci (Qassim) 2009;3:227.
[14] Fang Y, Gao T, Zhang B, Pu J. Recent Advances: Decoding Alzheimer’s Disease With Stem Cells. Front Aging Neurosci 2018;10. https://doi.org/10.3389/FNAGI.2018.00077.
[15] Pittenger MF, Discher DE, Péault BM, Phinney DG, Hare JM, Caplan AI. Mesenchymal stem cell perspective: cell biology to clinical progress. Npj Regen Med 2019 41 2019;4:1–15. https://doi.org/10.1038/s41536-019-0083-6.
[16] Xiong Y, Mahmood A, Chopp M. Angiogenesis, neurogenesis and brain recovery of function following injury. Curr Opin Investig Drugs 2010;11:298.
[17] Bonaventura G, Munafò A, Bellanca CM, La Cognata V, Iemmolo R, Attaguile GA, et al. Stem Cells: Innovative Therapeutic Options for Neurodegenerative Diseases? Cells 2021;10. https://doi.org/10.3390/CELLS10081992.
[18] Hernández AE, García E. Mesenchymal Stem Cell Therapy for Alzheimer’s Disease. Stem Cells Int 2021;2021. https://doi.org/10.1155/2021/7834421.
[19] Lübke JH, Idoon F, Mohasel-Roodi M, Alipour F, Hami J, Ehteshampour A, et al. Neurotrophic factors in Alzheimer’s disease: pathogenesis and therapy 2021. https://doi.org/10.21307/ane-2021-028.
[20] Wilkins A, Kemp K, Ginty M, Hares K, Mallam E, Scolding N. Human bone marrow-derived mesenchymal stem cells secrete brain-derived neurotrophic factor which promotes neuronal survival in vitro. Stem Cell Res 2009;3:63–70. https://doi.org/10.1016/J.SCR.2009.02.006.
[21] Moradi S, Mahdizadeh H, Šarić T, Kim J, Harati J, Shahsavarani H, et al. Research and therapy with induced pluripotent stem cells (iPSCs): social, legal, and ethical considerations. Stem Cell Res Ther 2019 101 2019;10:1–13. https://doi.org/10.1186/S13287-019-1455-Y.
[22] Joyce N, Annett G, Wirthlin L, Olson S, Bauer G, Nolta JA. Mesenchymal stem cells for the treatment of neurodegenerative disease. Regen Med 2010;5:933. https://doi.org/10.2217/RME.10.72.
[23] Gasiorowska A, Wydrych M, Drapich P, Zadrozny M, Steczkowska M, Niewiadomski W, et al. The Biology and Pathobiology of Glutamatergic, Cholinergic, and Dopaminergic Signaling in the Aging Brain. Front Aging Neurosci 2021;13:391. https://doi.org/10.3389/FNAGI.2021.654931/BIBTEX.
[24] Maloney JA, Bainbridge T, Gustafson A, Zhang S, Kyauk R, Steiner P, et al. Molecular mechanisms of Alzheimer disease protection by the A673T allele of amyloid precursor protein. J Biol Chem 2014;289:30990–1000. https://doi.org/10.1074/JBC.M114.589069.
[25] Muratore CR, Rice HC, Srikanth P, Callahan DG, Shin T, Benjamin LNP, et al. The familial Alzheimer’s disease APPV717I mutation alters APP processing and Tau expression in iPSC-derived neurons. Hum Mol Genet 2014;23:3523–36. https://doi.org/10.1093/HMG/DDU064.
[26] Ojeda-Hernández DD, Canales-Aguirre AA, Matias-Guiu J, Gomez-Pinedo U, Mateos-Díaz JC. Potential of Chitosan and Its Derivatives for Biomedical Applications in the Central Nervous System. Front Bioeng Biotechnol 2020;8:389. https://doi.org/10.3389/FBIOE.2020.00389/BIBTEX.
[27] Onyema HN, Berger M, Musyanovych A, Bantz C, Maskos M, Freese C. Uptake of polymeric nanoparticles in a human induced pluripotent stem cell-based blood–brain barrier model: Impact of size, material, and protein corona. Biointerphases 2021;16:021004. https://doi.org/10.1116/6.0000889.
[28] Essa D, Kondiah PPD, Choonara YE, Pillay V. The Design of Poly(lactide-co-glycolide) Nanocarriers for Medical Applications. Front Bioeng Biotechnol 2020;8. https://doi.org/10.3389/FBIOE.2020.00048.
[29] Annu, Sartaj A, Qamar Z, Md S, Alhakamy NA, Baboota S, et al. An Insight to Brain Targeting Utilizing Polymeric Nanoparticles: Effective Treatment Modalities for Neurological Disorders and Brain Tumor. Front Bioeng Biotechnol 2022;10:19. https://doi.org/10.3389/FBIOE.2022.788128/BIBTEX.
[30] Smith AO, Adzraku SY, Ju W, Qiao J, Xu K, Zeng L. A novel strategy for isolation of mice bone marrow endothelial cells (BMECs). Stem Cell Res Ther 2021;12. https://doi.org/10.1186/S13287-021-02352-3.
[31] Pacheco-Herrero M, Soto-Rojas LO, Reyes-Sabater H, Garcés-Ramirez L, De La Cruz López F, Villanueva-Fierro I, et al. Current Status and Challenges of Stem Cell Treatment for Alzheimer’s Disease. J Alzheimer’s Dis 2021;84:917–35. https://doi.org/10.3233/JAD-200863.