Clinical Trials: Failures and Challenges on the Path to Treating Alzheimer’s
Ronny Priefer, Professor, Massachusetts College of Pharmacy and Health Sciences University
Jinwoo Lee, Pharmacy Student, Massachusetts College of Pharmacy and Health Sciences University
This year marks the 30th anniversary of the approval of tacrine, the first drug targeting Alzheimer’s disease. Although not a curative agent, it provided some assistance to those afflicted with this devastating disease. This was followed by an almost two decade long era of despair, which may have finally concluded.
Alzheimer’s disease (AD) is a neurodegenerative disorder with symptoms of memory loss and cognitive disability. Typically, it begins with mild symptoms that progress to become more severe. AD is the 5th leading cause of death in the United States among people 65 or older. In 2020, 5.8 million Americans suffered from AD, and this is expected to rise to 14 million by 2060.1 In 2020, the cost of this disease was $159-215 billion, with an anticipated increase to $379-500 billion annually, by 2040.1 Medications for AD have been limited and traditionally have only help in improving patients’ quality of life by slowing the symptoms of disease, but not actually targeting the underlying cause.
The two essential pathophysiologies of AD are: 1) the accumulation of Amyloid Beta (Aβ) peptides and 2) hyperphosphorylated tau proteins.2 The former mechanism begins with the Amyloid Precursor Protein (APP), a transmembrane neuronal protein, which when cleaved by β- then γ-secretase, an Aβ fragment is formed which can stack, forming the characteristic Aβ plaques. This insoluble biomolecule ultimately leads to the neuronal functional decline. The latter mechanism involves the cytoskeletal tau protein. When tau is hyperphosphorylated it can detach, forming neurofibril tangles within the cell. This path can destroy cell signaling as well as rupture the cell membrane. To date, it has not been conclusively determined whether the Aβ plaques precede or succeed the tau neurofibril tangles.2
The first AD drug approved by the FDA was tacrine, in 1993. It is an acetylcholinesterase inhibitor (AchEi), which allows for an increase in the concentration of acetylcholine (Ach) in the synapses. Since Ach is a primary neurotransmitter for maintaining memory and cognitive ability, increasing its concentration is a logical treatment option. Subsequently, rivastigmine, galantamine, and memantine were approved within approximately 10 years of tacrine. They either work as AchEi or a NMDA receptor antagonist. However, over 10 years passed without a newly approved drug. Finally, Namzaric was brought to the market, which was simply a combination of donepezil and memantine. It was not until 2021, when the controversial drug, aducanumab was approved. This was quickly followed by lecanemab earlier this year. Both drugs are monoclonal antibodies designed to increase Aβ clearance, thus reducing plaque accumulation.2 Herein, is an overview of the clinical trials, their successes, and failures, of AD medications over the past 30 years (Figure 1).
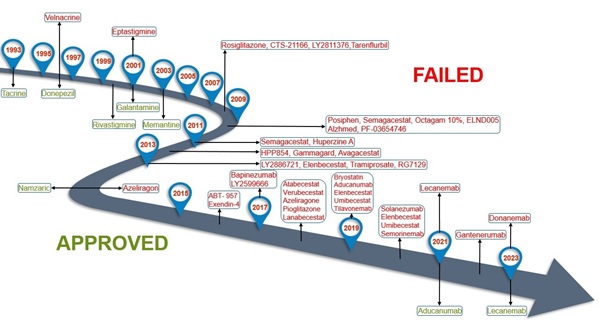
Figure 1. Overview of the clinical successes and failures of AD medications
EARLY SUCCESSES
Tacrine was the first approved AD medication, with a mode of action as an AchEi. It was marketed under the brand name, Cognex. Over fifteen clinical studies have been conducted to evaluate its efficacy, however the majority were of small sample size and short term. One notable study by Knapp et al, was a randomized, double-blinded, placebo-controlled, parallel-group trial with 263 elderly AD patients over a 30-week period.3 Patients were randomized into 4 groups with different administered doses of tacrine of up to 160mg/day, as well as placebo. It was ultimately determined that at the high doses, it was more effective than placebo in improving cognitive function.3 However, other studies indicated that tacrine could cause liver function damage, based on 50% of patients displaying alanine transaminase (ALT) levels higher than normal4,5. This demonstrated safety concern led to its withdrawal from the market in 2013.
In 1996, the second AchEi, donepezil, was approved under the brand name, Aricept. A thirty-week study has conducted with 473 mild to moderate AD patients.6 Patients were randomized into three groups, either 5 or 10mg/day of donepezil, or placebo. The Alzheimer’s Disease Assessment Scale-Cognitive subscale (ADAS-Cog) and the Clinician’s Interview-Based Impression of Change (CIBIC-plus) assessment tools were utilized. A higher score for either indicates greater cognitive impairment. It was reported that the mean differences in the ADAS-Cog, between donepezil and placebo groups, were 2.8 and 3.1, for the 5 and 10mg/day groups, respectively. In the CIBIC-plus, the mean differences were 0.35 and 0.39, respectively. These demonstrated that donepezil was statistically significantly, superior over the placebo, however no difference between the 5 and 10mg/day groups was observed.6 Likewise, in another fifteen-week study, the mean differences in ADAS-Cog were 2.7 and 3.0, for doses of 5 and 10mg/day, respectively.6 In Sweden, a randomized, double-blinded, placebo-controlled, clinical study was conducted among 248 moderate to severe AD patients.6 They began with 5mg of donepezil once daily for 28 days and titrated up to 10mg once daily. Severe Impairment Battery (SIB) and Alzheimer’s Disease Cooperative Study-Activities of Daily Living Inventory for Severe Alzheimer’s Disease (ADCS-ADL-severe) were utilized to assess the ability of donepezil to improve cognitive performance and daily function, respectively. A lower score suggests greater cognitive or functional impairment in both assessment tools. The mean differences between placebo and donepezil group were 5.9 and 1.8 points in each assessment, respectively; again, demonstrating superior over placebo.6
Rivastigmine, which is under the brand name, Exelon, is another AchEi and was approved in 2000. A 24-week, randomized, double-blinded, double-dummy, clinical study was conducted to assess changes in both cognitive performance and efficacy.7 ADAS-Cog was utilized to evaluate cognitive performance. In total, 1195 mild to moderate AD patients were randomized into 4 groups: 1) rivastigmine patch (9.5mg/daily), 2) rivastigmine patch (17.4mg/daily), 3) rivastigmine capsules (6mg/twice daily), or 4) placebo. When compared to the placebo group, the mean differences in ADAS-Cog were 1.8, 2.9, and 1.8, respectively. These differences supported the claim that rivastigmine was statistically significantly, superior over placebo.7 Another randomized, double-blinded, clinical trial was conducted over 24 weeks to compare the efficacy of rivastigmine patches, either 4.6 or 13.3mg/daily, in treating severe AD.7 In total, 716 patients were randomized into each group in a 1:1 ratio. SIB and Alzheimer’s Disease Cooperative Study-Activities of Daily Living-Severe Impairment Version (ADCS-ADL-SIV) were utilized to assess patient improvement. Both tools demonstrated that the latter was statistically significantly, superior over the latter.7
In 2001, another AchEi for AD, galantamine, was approved under the brand name, Razadyne. It has three different dosage forms: 1) extended release (ER) capsule, 2) immediate release (IR) tablet, and 3) an oral solution. In the United States, a 21-week study with 978 AD patients was conducted.8 Patients were randomized into 8, 16, or 24 mg/daily of galantamine IR tablet or placebo. ADAS-Cog and CIBIC-plus were utilized to assess the ability of galantamine to improve cognitive performance and clinical efficacy. The mean differences for the former tool were 1.7, 3.3, and 3.6, for the 8, 16, and 24mg/day groups, respectively. This revealed that galantamine IR was statistically significantly, superior over placebo, however there was no difference between the 16 and 24mg/day groups. Mean differences for the latter tool were 0.15, 0.41, and 0.44, for the 8, 16, and 24mg/day groups, respectively. Again indicating that galantamine was superior over placebo, with no clear difference between the 16 and 24mg/day groups.8 Yet another 26-week study with 636 patients was conducted to evaluate the efficacy of 24 and 32mg of galantamine IR. Mean differences in ADAS-Cog were 3.9 and 3.8 for the 24 and 32mg/day groups, respectively. Both doses were superior over placebo, but again no difference between the two groups was observed.8 To evaluate the efficacy of ER capsules, a randomized, double-blinded, placebo-controlled, clinical trial was conducted over 6 months.8 ADAS-Cog and CIBIC-plus were again employed as assessment tools, however only the former showed that galantamine ER capsule was superior over placebo.8 In the United States, a 21-week, randomized, clinical trial was conducted with 978 AD patients, utilizing the oral form of the AchEi.9 Patients were randomized into 8, 16, or 24mg/daily of galantamine oral solution or placebo. ADAS-Cog and CIBIC-plus were again utilized. The former assessment tools indicated the mean differences of 1.7, 3.3, and 3.6, respectively. The latter revealed that the mean differences were 0.15, 0.41, and 0.44, respectively. Both tools demonstrated superiority over placebo.9
Memantine, the first and only marketed NMDA receptor antagonist for AD, was approved in 2003. It was evaluated based on the hypothesis that the excitatory amino acid, glutamate, activates NMDA receptors which may lead to AD. In Latvia, a double-blinded, study was conducted for 12 weeks with 166 AD patients.10 Memantine dosing began at 5mg/day for 1 week and titrated up to once daily, 10mg. Behavioral Global Impression of Change (BGP) and Clinical Global Impression of Change (CGI-C) tools were utilized to evaluate day-to-day function and clinical benefits. Both demonstrated that memantine had statistically significant, benefits over placebo.10 Two additional notable clinical studies were conducted in the United States. Study 1 was 28 weeks with 252 moderate to severe AD patients.10 The assessment tool, ADCS-ADL was applied to evaluate day-to-day function while SIB was utilized to determine the ability of memantine to improve cognitive function. The mean differences between memantine and placebo groups in the two assessments were 3.4 and 5.7, respectively. Study 2 contained 404 moderate to severe AD patients already treated with donepezil.10 Patients were initially administered once daily, 5mg, and titrated up to 20mg/day, of memantine. ADCS-ADL and SIB revealed mean differences of 1.6 and 3.3, respectively, in the combination versus memantine and placebo group.10
Due to the aforementioned positive results with a donepezil/memantine, in 2014 a combination, under the brand name, Namzaric, was approved for moderate to severe AD. A 24-week, randomized, double-blinded, clinical trial was conducted with 677 AD patients who had been receiving the AchEi for at least 3 months, to evaluate the efficacy of memantine with donepezil.11 Approximately 70% of patients were receiving donepezil prior to start of the trial. SIB was applied to assess efficacy. The mean difference between groups was 2.6, which demonstrate that memantine with donepezil was superior over placebo with the AchEi. Additionally, CIBIC-plus was used to assess the clinical effect, revealing a mean difference of 0.3, again displaying superiority over placebo.11
ERA OF DISPAIR
After the successful development of AchEi and NMDA receptor antagonist, multiple clinical trials were conducted to develop new medications that target different pathways. These trials focused on the primary underlying causes of AD, such as Aβ and tau. Despite significant resources, multiple clinical trials failed due to a plethora of reasons. In 2008 alone, four clinical trials were terminated. Specifically, LY2811376, CTS-21166, tarenflurbil, and rosiglitazone all failed to advance. LY2811376 and CTS-21166 targeted β-secretase in hopes to reduce Aβ production.2 The clinical trial for the former was conducted with satisfactory pharmacokinetic and pharmacodynamic results. However, it was terminated as a result of a chronic toxicology study on a rat model, which revealed pathology issues of the retina and brain.12 A clinical trial on the latter medication was conducted with 56 patients.13 They were administered up to 225mg of CTS-21166 and reported significant inhibition effect. However, no further announcements on its advancing were made. Tarenflurbil targeted γ-secretase to reduce the production of long Aβ fragments. A randomized, double-blinded, 2-group, parallel study was conducted in the United States for 18 months, on patients with mild AD.14 Patients were randomized into doses of 800mg/day tarenflurbil or placebo. ADAS-Cog and ADCS-ADL score were utilized to evaluate efficacy. Unfortunately, no mean differences were reported with either assessment tool.14 Rosiglitazone aimed to increase clearance of Aβ and decrease plaque formation. A randomized, double-blinded, clinical trial was conducted to evaluate its efficacy.15 Mild to moderate AD patients were randomized into once daily, 4mg for a month, with subsequent increase to 8mg, once daily. Cerebral Metabolic Rate of Glucose (CMRglu) was utilized as an assessment tool, however it failed to demonstrate efficacy of rosiglitazone over placebo.15
Avagacestat, another γ-secretase inhibitor, failed in its clinical trials. In 2009, a randomized, placebo controlled, Phase 2, clinical trial with a parallel, untreated, non-randomized, observational cohort of CSF biomarker-negative individuals was conducted with 263 AD patients.16 Patients who were assigned avagacestat, began with 50mg/day for the first 2 weeks and titrated up to 125mg/day. Due to high rates of discontinuation, the dose was reduced back down to 50mg/day, and then further to 25mg/day. Alzheimer’s Disease Cooperative Study Mild Cognitive Impairment Activities of Daily Living (ADCS-MCI-ADL), ADAS-Cog, Clinical Dementia Rating Sum of Boxes (CDR-SB), and Mini-Mental State Examination (MMSE) were utilized to evaluate the efficacy of avagacestat. However, the mean differences showed no statistically significantly, superiority over placebo.16
In 2012, Phase 1 and 2 clinical studies of the β-secretase inhibitor, LY2886721, were conducted to evaluate its pharmacodynamics and efficacy.17 Patients, 55 or older, were randomized into once daily 15, 35, or 70mg of LY2886721, or placebo. However, these trials were terminated early due to four patients displaying an abnormal liver function test.17
Another approach utilized anti-tau IgG4 antibodies, which bind to the N-terminus of extracellular tau. A randomized, double-blinded, placebo-controlled, clinical study with 453 AD patients was conducted to evaluate the efficacy and safety of the biologic, tilavonemab.18 Patients were randomized into 300, 1000, or 2000mg of tilavonemab every 4 weeks. The study utilized the CDR-SB assessment to evaluate efficacy. However, in 2019, it was reported that tilavonemab was not significant superiority over placebo.18 Another randomized, double-blinded, placebo-controlled, Phase 2 clinical study with another IgG4 antibody, semorinemab, was conducted on mild AD patients to evaluate its efficacy and safety.19 Patients were randomized into semorinemab, or placebo group. CDR-SB, percentage of adverse events, Columbia Suicide Severity Rating Scale (C-SRRS), and abnormal MRI findings were utilized as assessment tools. Unfortunately, the clinical trial failed to meet its endpoint, with no evidence of superiority over placebo.19
RAGE is a receptor on the cell-surface that regulates the accumulation and formation of Aβ plaques. Azeliragon was investigated as an approach to decrease the formation as well as increase the clearance of these plaques. In 2007, a randomized, double-blinded, placebo-controlled, clinical trial was conducted with 399 AD patients to evaluate safety, tolerability, and efficacy of azeliragon.20 Patients were randomized into doses of 15 or 60mg/daily of azeliragon, or placebo. ADAS-Cog and Report of Adverse Events were utilized as assessment tools. The high dose treatment was discontinued due to adverse effects, including confusion, falls, and CNS toxicity. Ultimately, outcome measurements demonstrated no significant superiority over placebo.20 Similarly, a subsequent randomized, double-blinded, placebo-controlled, clinical trial was conducted on 880 mild AD patients to evaluate 5mg once daily of azeliragon.21 ADAS-Cog and CDR-SB were utilized as assessment tools. Again, this clinical trial failed due to lack of efficacy.21
Aducanumab, is a humanized IgG1, anti-monoclonal antibody that targets the clearance of Aβ. A Phase 3, multicentered, double-blinded, placebo-controlled, parallel-group, clinical study was conducted with 1653 AD patients to evaluate its efficacy and safety.22 Patients were randomized into low or high dose of aducanumab, or placebo. CDR-SB and MMSE were utilized as assessment tool, however, no superiority was demonstrated.22 Despite this, further analysis suggested positive results and thus Phase 3 studies were conducted.
IS THE FUTURE NOW?
Finally in 2021, aducanumab was approved under the brand name Aduhelm, which became the first medication that specifically targeted the underlying cause of AD.23 A study with 1638 AD patients, randomized into low or high doses of aducanumab, or placebo was conducted. CDR-SB and MMSE were utilized to measure the drug’s efficacy. Both assessments demonstrated that the high dose was significantly superior over placebo.23 In another study, 1647 AD patients were randomized into low or high dose of aducanumab, or placebo. Here however, no superiority was demonstrated when assessing with CDR-SB.23
Earlier this year a new biologic drug, lecanemab, was approved under the brand name, Leqembi. A double-blinded, placebo-controlled, parallel-group, dose-finding study was conducted with 1856 AD patients.24 Patients were randomized into 5 different doses of lecanemab, or placebo. CDR-SB, MMSE, and ADAS-Cog scores were utilized as the primary endpoint at week 53. Lecanemab demonstrated a 64% likelihood of a 25% or greater slowing of the progression of symptoms of AD. However, the success criteria was initially set at 80%. Amyloid PET Standard Uptake Value Ratio (PET SUVR), which consists of CDR-SB and ADAS-Cog, was utilized as a secondary efficacy point at week 79. Here, lecanemab failed to demonstrate superiority over placebo.24 However, both aducanumab and lecanemab were FDA approved via an accelerated program. During this approval process, a surrogate, or an intermediate endpoint was applied.25 currently, both are undergoing Phase 4, confirmatory trials, to demonstrate long-term benefits and risks.26
CONCLUSION
While there has been significant progress, both in past clinical trial results, and more recently in the development of AD treatment, challenges remain. Current drugs focus on slowing the progression of symptoms, while the ultimate goal with the newer medications is to treat the underlying causes. The recent approval of aducanumab and lecanemab offers hope; though Phase 4 clinical trials are still ongoing. Numerous other trials are underway which target different modes of action associated with AD. The continuously rising cost of healthcare is an issue that all stakeholders must consider when assessing patients’ access to treatments. Nevertheless, past failures and ongoing trials inform the scientific and pharmaceutical communities, and will hopefully lead to promising novel treatment options for AD patients in our near future.
References
1. What is Alzheimer’s Disease? Centers for Disease Control and Prevention https://www.cdc.gov/aging/aginginfo/alzheimers.htm. Published October 26,2020. Accessed February 17, 2023.
2. Asher S, Priefer R. Alzheimer’s disease failed clinical trials. Life Sciences. 2022;306: 120861.doi: 10.1016/j.lfs.2022.120861
3. Knapp MJ, Knopman DS, Solomon PR, et al. A 30-week randomized controlled trial of high dose tacrine in patients with Alzheimer’s disease. JAMA: The Journal of the American Medical Association. 1994;271 (13): 985-991.doi: 10.1001/jama.1994.03510370037029
4. Finley R. Tacrine for Alzheimer’s Disease: Therapeutic Considerations. Journal of Pharmacy Practice. 1995;8(5):217-227. doi:10.1177/089719009500800505
5.Watkins PB, Zimmerman HJ, Knapp MJ, et al. Hepatotoxic effects of tacrine administration in patients with Alzheimer’s disease. JAMA: The Journal of the American Medical Association. 1994;271(13):992-997. doi:10.1001/jama.1994.03510370044030
6. Aricept (donepezil hydrocholride) package insert. Eisai Inc. https://www.accessdata.fda.gov/drugsatfda_docs/label/2012/020690s035,021720s008,022568s005lbl.pdf
7. Exelon patch (rivastigmine) package insert. Novartis pharmaceutical corporation. https://www.novartis.com/us-en/sites/novartis_us/files/exelonpatch.pdf
8. Razadyne (galantamine hydrobromide) package insert. Janssen Pharmaceutical Inc. https://www.accessdata.fda.gov/drugsatfda_docs/label/2015/021615s021lbl.pdf
9. Galantamine hydrobromide oral solution package insert. Roxane laboratories Inc.https://www.accessdata.fda.gov/drugsatfda_docs/label/2009/078185s000lbl.pdf
10. Namenda (memantine hcl) package insert. Forest laboratories. https://www.accessdata.fda.gov/drugsatfda_docs/label/2013/021487s010s012s014,021627s008lbl.pdf
11. Namzaric (memantine hcl extended release and donepezil hcl) package insert. Forest laboratories. https://www.accessdata.fda.gov/drugsatfda_docs/label/2014/206439lbl.pdf
12. Vassar R. BACE 1 inhibitor drugs in clinical trials for Alzheimer’s disease. Alzheimer’s Research&Therapy.2014;6(89):1-14. doi: 10.1186/s13195-014-0089-7
13. Ghosh A, Brindisi M, Tang J, et al: Developing β-secretase inhibitors for treatment of Alzheimer’s disease. Journal of Neurochemistry. 2012;120(suppl1): 71-83.doi: 10.1111/j.1471-4159.2011. 07476.x
14. Green R, Schneider L, Amato D, et al. Effect of Tarenflurbil on Cognitive Decline and Activities of Daily Living in Patients with Mild Alzheimer Disease. JAMA. 2009;302(23): 2557-2564.doi:10.1001/jama.2009.1866
15. Effects of Avandia on Cognition and Cerebral Glucose Utilization in Subjects with Mild to Moderate Alzheimer's Disease (AD). Clinical trials.gov. Published October, 2020. https://clinicaltrials.gov/ct2/show/nct00265148
16. Coric V, Salloway S, Van Dyck, et al. Targeting Prodromal Alzheimer Disease with Avagacestat: A Randomized Clinical Trial. JAMA Neurology. 2015;72(11): 1324-1333.doi: 10.1001/jamaneurol.2015.0607
17. Assessment of Safety, Tolerability, and Pharmacodynamic Effects of LY2886721 in Patients with Mild Cognitive Impairment Due to Alzheimer's Disease or Mild Alzheimer's Disease. Clinical trials.gov. Published May,2018 https://clinicaltrials.gov/ct2/show/record/NCT01561430.
18. A Phase 2 Multiple Dose, Multicenter, Randomized, Double-Blind, Placebo-Controlled Study to Evaluate the Efficacy and Safety of ABBV-8E12 in Subjects with Early Alzheimer's Disease. Clinical trials.gov. Published August, 2022 https://clinicaltrials.gov/ct2/show/record/NCT02880956
19. A Phase II, Multicenter, Randomized, Double-Blind, Placebo-Controlled, Parallel-Group, Efficacy, and Safety Study of MTAU9937A in Patients with Prodromal to Mild Alzheimer's Disease. Clinical trials.gov. Published February,2022 https://clinicaltrials.gov/ct2/show/record/NCT03289143
20. Galasko D, Bell J, Mancuso J, et al. Clinical Trial of an inhibitor of RAGE-Aβ interactions in Alzheimer disease. American Academy of Neurology. 2014;82(17): 1536-1542.doi: 10.1212/. wnl.0000000000000364.
21. Randomized, Double-blind, Placebo Controlled, Multi-center Registration Trial to Evaluate the Efficacy and Safety of Azeliragon (TTP488) in Patients with Mild Alzheimer's Disease Receiving Acetylcholinesterase Inhibitors and/or Memantine. Clinical trials.gov. Published May, 2021 https://clinicaltrials.gov/ct2/show/record/NCT02080364
22. A Phase 3 Multicenter, Randomized, Double-Blind, Placebo-Controlled, Parallel-Group Study to Evaluate the Efficacy and Safety of Aducanumab (BIIB037) in Subjects with Early Alzheimer's Disease.clinical trials.gov. Published August,2021 https://clinicaltrials.gov/ct2/show/record/NCT02477800
23. Aduhelm (aducanumab) package insert. Biogen. https://www.accessdata.fda.gov/drugsatfda_docs/label/2021/761178s000lbl.pdf
24. Leqembi (lecanemab) package insert. Eisai Inc. https://www.accessdata.fda.gov/drugsatfda_docs/label/2023/761269s000lbl.pdf
25. Accelerated Approval. U.S. Food and Drug Administration. https://www.fda.gov/patients/fast-track-breakthrough-therapy-accelerated-approval-priority-review/accelerated-approval. Accessed March 15, 2023
26. FDA’s Decision to Approve New Treatment for Alzheimer’s Disease. U.S. Food and Drug Administration, https://www.fda.gov/drugs/news-events-human-drugs/fdas-decision-approve-new-treatment-alzheimers-disease. Accessed March 15, 2023














