

In the past few decades, biologics have re-shaped the treatment of numerous diseases: Innovative approaches like CAR-T cell therapy in fighting cancer have become well-established treatment options but require advanced biotechnological production processes.
One of the most essential vehicles to produce biologics for research or clinical applications are cells: They can either be used to express biologics of choice, or as therapeutics themselves – e.g. in the case of stem cell therapies or the Chimeric Antigen Receptor T (CAR-T) cell therapy. This makes it all more crucial to improve speed and quantity in cell culturing, without compromising on quality. Sophisticated bioprocessing is necessary to intensify the seed train, requiring that state-of-the-art techniques and technologies go hand in hand.
Seed train intensification includes the process of cultivating growing numbers of cells in vitro. This upstream processing step aims to efficiently leverage a relatively small amount of cells (e.g. provided by cell banks that had previously subjected them to cryogenic freezing procedures) in order to gain a sufficient quantity for further processing.
A seed train usually involves repetitive cultivation phases of cells over several days, after each of which they are transferred into larger containers until a sufficient amount of biomass is obtained. Once this has been achieved, the cells can be used for the inoculation of a production bioreactor, followed by monoclonal antibody production, gene expression, or several other biotechnological processes.1
A traditional seed train starts with thawing very small volumes of cells from a master cell bank – often around only 1 ml. Once they have been fed by a sufficient degree, the cells are repeatedly transferred into containers like shake flasks, rocking bioreactors, and stirred tank bioreactors – with an increase in container volume at every step.
Towards the end, the original 1 ml cell culture may have achieved a volume of up to 2,000 L in single-use bioreactors, or even 15,000 L in stainless-steel bioreactors. The increased volume of such a working cell bank now allows the inoculation of a production bioreactor, eventually followed by further processing steps for providing access to biopharmaceutical products of different kinds.2 3 4
The repetitive transfer of growing cell volumes is not only laborious, but also time-consuming. Furthermore, the open handling of cell cultures poses the risk of contamination and alterations, consequently results in quality reduction, product loss, and further delays.
As already mentioned, conventional seed trains demand significant amounts of time and resources. Additionally, they often involve open processing of cells at several culturing levels – human error and contamination may put the entire seed train at risk.
This is in stark contrast to the time-critical need for sophisticated biologics in research, diagnostic, and clinical applications. Extended production times, unplanned delays due to product loss, or batch-to-batch inconsistencies are drawbacks that biopharma companies cannot afford: Not only does this endanger their commercial success – patients with the urgent need for novel therapeutics are put at an unjustifiable risk.
Investing in new approaches to future-proof and intensify the seed train can also be justified by the expected growth of the biopharmaceutical market: Already in 2018, more than 32% of the pharmaceutical sales in the U.S. come from biological medicines. Globally speaking, the biopharmaceutical market is expected to have a compound annual growth rate of 8% between 2024 and 2029. This underlines that it is high time to adjust dated production methods to meet growing demands.
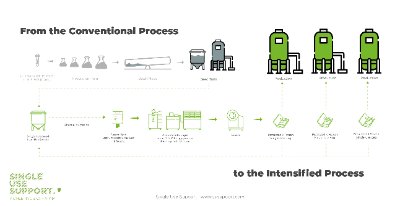
Figure 1: Advanced cell banking in the context of seed train intensification by Single Use Support
Seed train intensification is a great opportunity to speed up bioprocessing to meet the growing demand for biologics. Single Use Support has therefore innovated the process of cell expansion and intermediate cell banking with state-of the-art technologies, allowing for shortened production times while reducing the risk of failure.
Seed train intensification with Single Use Support is based on the cryopreservation of high density cell cultures: Multiple smaller units are filled from the seed train into single-use bags, which are subsequently subjected to cryogenic freezing with high precision. To this end, Single Use Support provides RoSS.LN2F – a cryogenic freezer that allows controlled freezing rates down to -170°C and can therefore cater to the hurdles in freezing cells at high density. Aliquoted and frozen, the banked single cell culture units can each be used for directly inoculating a production bioreactor – independently, without the need to restart the entire seed train from the beginning.
However, cryopreservation is not the only critical step in seed train intensification: Precision in fluid management as well as temperature control and bag-to-bag consistency of cell counts helps advance the liquid transfer in production. After all, these factors are essential to maintain cell viability and product consistency.
Single Use Support’s solutions are powerful options to significantly speed up the seed train while enhancing scalability and flexibility. Additionally, the risk of contamination as well as production costs can be reduced. Thus, seed train intensification with Single Use Support can be a feasible solution for biopharma companies to prepare themselves for tomorrow’s challenges and requirements in bioprocessing.
References:
1. Hernández Rodríguez T, Pörtner R, Frahm B. Seed train optimization for suspension cell culture. BMC Proc. 2013 Dec 4;7(Suppl 6):P9. doi: 10.1186/1753-6561-7-S6-P9. PMCID: PMC3981631.
2. Eder M. Seed train intensification - the next big step in biopharma? July 2020. https://www.susupport.com/knowledge/cell-gene-therapy/seed-train-intensification-next-step-biopharma?rel=search#Seed%20train%20-%20the%20conventional%20process.
3. Statista. Biologics share of total pharmaceutical sales in select countries in 2018. May 2020. https://www.statista.com/statistics/1118421/biologic-medicines-share-of-total-pharmaceutical-sales-in-oecd-countries/
4. Mordor Intelligence. Biopharmaceutical Industry Size & Share Analysis - Growth Trends & Forecasts (2024 - 2029). https://www.mordorintelligence.com/industry-reports/global-biopharmaceuticals-market-industry